Abstract
Glioependymal cyst (GEC) is an uncommonly observed clinical entity in the posterior cranial fossa. A 36-year-old female with cystic lesion in the right cerebellum was hospitalized for evaluating headache and dizziness. Brain images showed a well-defined, ovoid mass adjacent to the fourth ventricle. After drainage and excision of the cyst, the patient became symptom free. Pathology examination disclosed low cuboidal epithelium and glial cells in the cyst wall. The radiological features, neurological manifestations, and the operations for GECs of the present localization are described in this short communication.
Glioependymal cysts (GECs) are benign neuroepithelial lesions that have been infrequently detected; they are also known as neuroglial cysts. These developmental lesions virtually occur everywhere within the brain and spinal cord, and represent less than 1% of primary intracranial cysts [1]. They might virtually present at intraparenchymal, intraventricular, subarachnoid, intraneural, and intraspinal regions [234]. The reported intracerebral GECs are predominantly originated in the central white matter of the frontal lobe [5]. For this reason, there is a lack of available information about GECs of the infratentorial compartment in the relevant literature [67].
The author herein illustrates an interesting case harboring a GEC developed in the cerebellum near the fourth ventricle. The present report also provides the histogenesis, radiographic findings, and pathological details for differentiating this rare type from other cysts more common in the posterior cranial fossa.
This 36-year-old lady had a 3-month history of occipital headache and worsening dizziness. On admission, the only abnormal feature at the physical examination was a mild gait ataxia. Head CT showed a homogenous, low attenuated mass with smooth border in the right cerebellum. Preoperative MRI revealed a 25×23×22 mm sized intraparenchymal cyst with minimal pressure effect on the surrounding structures. The intracystic fluid collection was isointense with cerebrospinal fluid on all MR sequences. There was no rim or nodular enhancement after infusion of contrast agent (Fig. 1A−C).
Given the progression of symptoms, the surgeon carried out a suboccipital craniotomy and transcortical approach to the lesion. Intraoperatively, opening the cyst wall immediately produced a clean fluid. The cyst buried within cerebellar white matter, and was separated from the fourth ventricle. The mass was totally extirpated. The patient tolerated the operation well without neurological morbidities. At the 4-week follow up appointment, she had complete resolution of preoperative symptoms and signs. There has been no evidence of cyst recurrence on repeated CT scan 15 years after the intervention (Fig. 1D).
Pathological study disclosed that the cyst consisted of an inner layer of cuboidal ependymal cells and an external layer of glial tissues. An immunopositivity for glial fibrillary acidic protein and S-100 protein in the cells supported the diagnosis of a GEC (Fig. 2).
The IRB exempted informed consent due to its retrospective nature and minimal risk for harm to the patient, and this report was conducted according to the guidelines of the Declaration of Helsinki for biomedical research.
The posterior cranial fossa is an extremely rare site for GECs that leaded to clinical manifestations. To the best of the author's knowledge, only 11 cases with these cysts pathologically diagnosed have been referenced in the related literature (Table 1) [8,9,10,11,12,13,14]. Six cysts were observed in the cerebellopontine angle region, three at the midbrain, and two entirely within cerebellar hemisphere. No cysts involved the cavity of the fourth ventricle. The author confirmed that most cases were women and their average age was 42.3 years. And, those of the prior reports are cysts with various sizes seen in adult presentation.
The cysts are congenital lesions, arising from rests of embryonic neural tube segments that become sequestered within the developing white matter [2151617]. These ectopic cells thus evolve into epithelial-lined cysts that are located intracerebrally, extracerebrally or anywhere along the neural axis and do not communicate with the ventricular system. Consequently, almost all cases reported have exhibited a propensity for the periventricular location within the supra- and infra-tentorial compartments for their given cysts [14].
They usually contain cerebrospinal fluid-like clear fluid; however different aspects of intracavitary contents (xanthochromic, opalescent, milky, and turbid) have been described in the reports [118]. In pathological evaluation, they reveal the partly ciliated cuboidal or columnar lining epitheliums resting directly over the astroglial tissue bundles without an intervening basal membrane [19]. The immunohistochemistry depicts that the surface-lining cells are positively stained for glial fibrillar acidic protein and S-100 protein, and are negative for epithelial membrane antigen, cytokeratin, and carcinoembryonic antigen [816]. This specificity suggests that GECs have an origination from the neural ectoderm. Both ependymal cyst and choroid plexus cyst have the similar characteristics to the current cyst in the immunohistochemistry, so that it is important to notion that the expression of the glial stroma is a decisive to accurately find out the GEC from other nontumorous cysts.
Mostly, GECs are clinically silent; however, in certain cases, they cause symptoms and require treatment. Due to the secretory nature of the epithelial cells, these cysts can grow to sizable masses over time and progressively result in the signs and symptoms [20]. Generally, the neurologic deficits depend on the locations and expansion of the GECs. The adult supratentorial cysts have presented with a varying symptomatology [212223]. The posterior fossa lesions more commonly have induced dizziness, double vision, syncope, gait disturbance, cranial neuropathy, and hemifacial spasm [6789]. Characteristically, those presentations were linked to the grade of obstruction of the cerebrospinal fluid flow, severity of intracranial hypertension, and degree of compression of the neural-vascular structures including the brain stem, cerebral aqueduct, cerebellum, and the fourth ventricle.
Typically, CT scans depicted unilocular cyst with thin-walled morphology [5]. On MR images, the cysts have smooth border and their contents do not restrict in diffusion weighted sequence [724]. In radiological differentiation from GECs, scalloping of the adjacent cranium is often seen in the arachnoid cysts [25]. However, the most difficult lesion to distinguish from the GEC is an arachnoid cyst, especially when it occupies the cerebellopontine angle. The contents of epidermoid and dermoid cysts were positive with diffusion weighted MRI and heterogenous on fluid attenuated inversion recovery image. Furthermore, typically both grow larger with insinusating pattern [26]. Next, porencephalic cysts communicate with the lateral ventricle and show surrounding gliosis and spongiosis [27]. Contrary to GECs, enlarged perivascular spaces are typically multiple and cluster around the basal ganglia. Infectious cyst, such as neurocysticercosis, is commonly smaller than 10 mm and partly calcified and enhances [19]. Neurenteric cysts are characterized for isointensity to hyperintensity on T1-weighted MRI [6]. Lastly, ependymal cyst may be indistinguishable from GECs; however this is frequently detected as an intraventricular mass within the lateral ventricles [26].
The operative approaches are selected according to the cyst locations and their proximity to subarachnoid or ventricular spaces. Numerous procedures have been introduced, including trephination and aspiration, craniotomy and excision, fenestration into the cistern, open or endoscopic cystoventriculostomy, and cystoperitoneal, cystocisternal or cystoventricular shunting [11322]. Recently, endoscopic fenestration of the cerebral hemispheric cysts thorough the adjacent ventricle is done as the first line of treatment for the selected cases to avoid the craniotomy and shunt dependence [122328]. Simple puncture and drainage by using the open or stereotaxy has a higher recollection rate of the cysts [29]. In light of such evidences, complete resection is the best mode of treatment whenever possible, especially for symptomatic cerebellar GECs at an early stage of the disease. Interestingly, six reports have described GECs of the cerebellopontine angle cistern, three of which presented with hemifacial spasms and they underwent the total or partial excision with microvascular decompression, resulting in long-term resolution of symptoms [1011]. For the cysts in the dorsal midbrain with hydrocephalus, adding third ventriculostomy, aqueductal stenting or ventriculoperitoneal shunt might to lessen the possible risk for recurrence in cerebrospinal fluid circulation disturbance.
In summary, the presented reporting highlights that the cerebellum is an unusual location for intraparenchymal GECs. Despite of its rarity, this congenital condition should be considered a potential diagnosis for the cysts either midline or at cerebellopontine angle in the posterior fossa. Complete cystectomy is curative operation for cerebellar GECs with neurological symptoms and sings.
References
1. Robles LA, Paez JM, Ayala D, Boleaga-Duran B. Intracranial glioependymal (neuroglial) cysts: a systematic review. Acta Neurochir (Wien). 2018; 160:1439–1449. PMID: 29802560.
2. Schelper RL, Ramzy I, Durr N. Ependymal cyst of the subarachnoid space. Cytologic diagnosis and developmental considerations. Acta Cytol. 1985; 29:44–48. PMID: 3855586.
3. Cavalheiro S, Canullo ML, Silva da Costa MD, Dastoli PA, Mendonça Nicácio J, Stavale JN. Glioependymal cyst on the third cranial nerve: case report. J Neurosurg Pediatr. 2020; 25:178–182.
4. Saito K, Morita A, Shibahara J, Kirino T. Spinal intramedullary ependymal cyst: a case report and review of the literature. Acta Neurochir (Wien). 2005; 147:443–446. discussion 446. PMID: 15666187.
5. Qi X, Xie D, Wan Y, et al. Glioependymal cyst of frontal lobe. Neurosurg Q. 2015; 25:280–282.
6. Monaco P, Filippi S, Tognetti F, Calbucci F. Glioependymal cyst of the cerebellopontine angle. J Neurol Neurosurg Psychiatry. 1995; 58:109–110.
7. Elmadbouh H, Halpin SF, Neal J, Hatfield RH, Hourihan MD. Posterior fossa epithelial cyst: case report and review of the literature. AJNR Am J Neuroradiol. 1999; 20:681–685. PMID: 10319981.
8. Ho KL, Chason JL. A glioependymal cyst of the cerebellopontine angle. Immunohistochemical and ultrastructural studies. Acta Neuropathol. 1987; 74:382–388. PMID: 3500568.
9. Tsuchida T, Kawamoto K, Sakai N, Tsutsumi A. Glioependymal cyst in the posterior fossa. Clin Neuropathol. 1997; 16:13–16. PMID: 9020388.
10. Harada A, Takeuchi S, Inenaga C, et al. Hemifacial spasm associated with an ependymal cyst in the cerebellopontine angle. J Neurosurg. 2002; 97:482–485. PMID: 12186482.
11. Shenouda EF, Moss TH, Coakham HB. Cryptic cerebellopontine angle neuroglial cyst presenting with hemifacial spasm. Acta Neurochir (Wien). 2005; 147:787–789. discussion 789. PMID: 15900403.
12. Endo H, Fujimura M, Watanabe M, Tominaga T. Neuro-endoscopic management of mesencephalic intraparenchymal cyst: a case report. Surg Neurol. 2009; 71:107–110. discussion 110. PMID: 18291476.
13. Park BJ, Kim YI, Jeun SS, Lee YS. An ependymal cyst in cerebello-pontine angle presenting with syncope. Brain Tumor Res Treat. 2013; 1:121–123. PMID: 24904905.
14. Prieto R, Subhi-Issa I, Pascual JM. Ependymal cyst of the midbrain. Clin Neuropathol. 2013; 32:183–188. PMID: 23254139.
15. Andrews BT, Halks-Miller M, Berger MS, Rosenblum ML, Wilson CB. Neuroepithelial cysts of the posterior fossa: pathogenesis and report of two cases. Neurosurgery. 1984; 15:91–95. PMID: 6332281.
16. Cho HJ, Kim HN, Kim KJ, et al. Intracranial extracerebral glioneuronal heterotopia with adipose tissue and a glioependymal cyst: a case report and review of the literature. Korean J Pathol. 2014; 48:254–257. PMID: 25013427.
17. Vasquez CA, Casey M, Folzenlogen Z, Ormond DR, Lillehei K, Youssef AS. Third ventricular cerebrospinal fluid cysts of thalamic origin: review of embryologic origin, presentation, and management strategies with a case series. World Neurosurg. 2017; 103:210–219. PMID: 28391023.
18. Alvarado AM, Smith KA, Chamoun RB. Neuroendoscopic fenestration of glioependymal cysts to the ventricle: report of 3 cases. J Neurosurg. 2019; 131:1615–1619.
19. Goh RH, Maguire J. Neuroepithelial cyst of the posterior fossa: two case reports with radiologic-pathologic correlation. Can Assoc Radiol J. 1996; 47:126–131. PMID: 8612085.
20. Umredkar A, Mohindra S, Gupta R, Bal AK. Contrasting behavior of glio-ependymal cysts: a report of two cases and literature review. Neurol India. 2010; 58:659–661. PMID: 20739818.
21. Heran NS, Berk C, Constantoyannis C, Honey CR. Neuroepithelial cysts presenting with movement disorders: two cases. Can J Neurol Sci. 2003; 30:393–396. PMID: 14672275.
22. Ferreira T, Khurana D, Mohindra S, Gupta K. Hemiatrophy as a presentation of a glioependymal cyst. Clin Neurol Neurosurg. 2014; 124:6–7. PMID: 24976022.
23. El Damaty A, Marx S, Fleck S, Schroeder HW. Neuroendoscopic approach to intracranial ependymal cysts. World Neurosurg. 2017; 97:383–389. PMID: 27751924.
24. Hasegawa H, Ushio Y, Oku Y, Iwata Y, Kanai N, Kamikawa K. Neuroepithelial cyst of the cerebellar vermis. Surg Neurol. 1976; (3):181–184. PMID: 959991.
25. Markwalder TM, Markwalder RV, Slongo T. Intracranial ciliated neuroepithelial cyst mimicking arachnoid cyst. Surg Neurol. 1981; 16:411–414. PMID: 7330761.
26. Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology. 2006; 239:650–664. PMID: 16714456.
27. Boockvar JA, Shafa R, Forman MS, O'Rourke DM. Symptomatic lateral ventricular ependymal cysts: criteria for distinguishing these rare cysts from other symptomatic cysts of the ventricles: case report. Neurosurgery. 2000; 46:1229–1232. PMID: 10807256.
28. Conrad J, Welschehold S, Charalampaki P, van Lindert E, Grunert P, Perneczky A. Mesencephalic ependymal cysts: treatment under pure endoscopic or endoscope-assisted keyhole conditions. J Neurosurg. 2008; 109:723–728. PMID: 18826361.
29. Lee SJ, Hong CK, Ahn JY, Lee KS. Progressively enlarged intracerebral ependymal cyst presenting with movement disorder. J Korean Neurosurg Soc. 2007; 41:252–254.
Fig. 1
Radiology of glioependymal cyst in the cerebellum. A and B: The signal intensities of lesion are identical to cerebrospinal fluid in both T2-weighted and fluid attenuated inversion recovery MRI images. C: No abnormal enhancement is observed after gadolinium administration. D: Dynamic CT taken 15 years after the surgery depicts disappearance of the lesion.
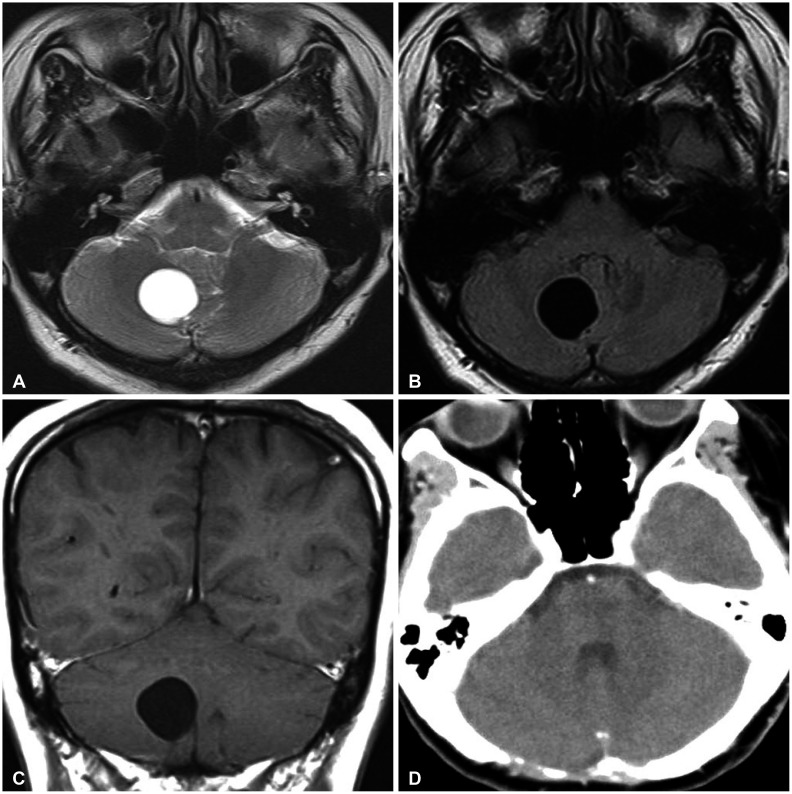
Fig. 2
Microphotography exhibits stratified flattened cuboidal epitheliums and underlying neuroglial tissue in the cyst wall (H&E, ×200).
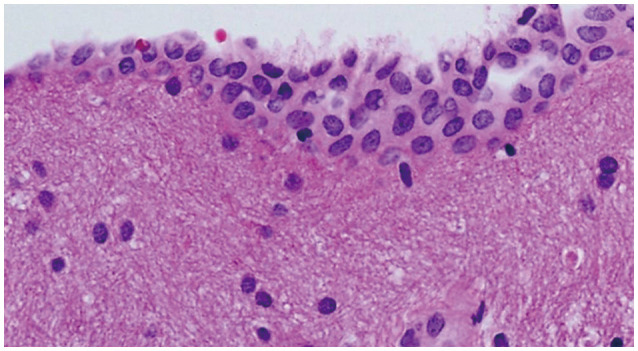
Table 1
Reported adult cases of glioependymal cysts in the posterior fossa
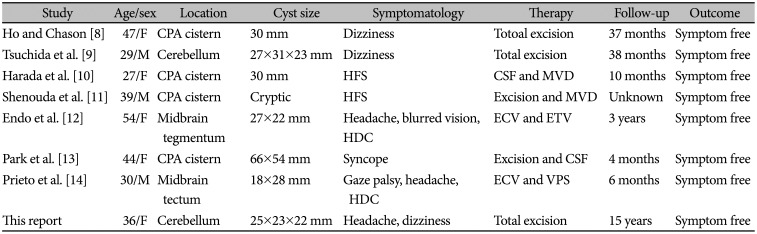
| Study | Age/sex | Location | Cyst size | Symptomatology | Therapy | Follow-up | Outcome |
|---|---|---|---|---|---|---|---|
| Ho and Chason [8] | 47/F | CPA cistern | 30 mm | Dizziness | Totoal excision | 37 months | Symptom free |
| Tsuchida et al. [9] | 29/M | Cerebellum | 27×31×23 mm | Dizziness | Total excision | 38 months | Symptom free |
| Harada et al. [10] | 27/F | CPA cistern | 30 mm | HFS | CSF and MVD | 10 months | Symptom free |
| Shenouda et al. [11] | 39/M | CPA cistern | Cryptic | HFS | Excision and MVD | Unknown | Symptom free |
| Endo et al. [12] | 54/F | Midbrain tegmentum | 27×22 mm | Headache, blurred vision, HDC | ECV and ETV | 3 years | Symptom free |
| Park et al. [13] | 44/F | CPA cistern | 66×54 mm | Syncope | Excision and CSF | 4 months | Symptom free |
| Prieto et al. [14] | 30/M | Midbrain tectum | 18×28 mm | Gaze palsy, headache, HDC | ECV and VPS | 6 months | Symptom free |
| This report | 36/F | Cerebellum | 25×23×22 mm | Headache, dizziness | Total excision | 15 years | Symptom free |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download