Abstract
Appendiceal cancer is an extremely rare malignancy, and its metastatic spread to the brain is even more unusual. We describe a 47-year-old female who presented with a rare cerebral appendiceal carcinoma metastasis, a case that is further remarkable for representing the first histologic diagnosis of primary medullary carcinoma in the appendix. Based on a comprehensive review of the English literature using PubMed, Embase, and Google Scholar, only six other cases of cerebral appendiceal metastases have been described.
Appendiceal cancer is an unusual neoplasm with an annual incidence of 1.3 cases per 1,000,000 people [1]. Nonperitoneal metastases are observed at presentation in approximately 20% of cases with the liver and lung accounting for the majority of sites [2]. Superimposed on the rarity of appendiceal cancer is the rarity of its metastasis to the brain. In an eight-year Surveillance, Epidemiology, and End Results (SEER) database analysis, Thompson et al. [3] identified 3 cases of brain metastases among 7,215 cases of appendiceal cancer for a prevalence of 0.042%. Habbous et al. [4] estimated in a nine-year population study that appendiceal primaries accounted for <0.02% of all brain metastases. Few case reports have described its intracranial spread [56].
Consequently, the nature of appendiceal cancer metastatic to the brain is poorly understood. In this report, we review our experience with a 47-year-old female who presented with a rare cerebral appendiceal carcinoma metastasis. This case may also represent the first diagnosis of primary medullary carcinoma of the appendix. Further, we report our findings from the first comprehensive literature review performed for cerebral appendiceal carcinoma metastases.
A 47-year-old female with no significant past medical history presented in March 2019 to the emergency department following one week of nausea, vomiting, and headache. The remainder of her neurologic exam was negative. Upon further inquiry, she endorsed one year of abdominal pain associated with 13.6 kg unintentional weight loss. Imaging of the chest, abdomen, and pelvis demonstrated extensive disease involving the liver, spleen, left adrenal gland, mesentery, peritoneum, and abdominal wall as well as regional lymphadenopathy. Brain MRI demonstrated an isolated 3.6×3.3×3.2 cm heterogeneously enhancing right frontal mass with extensive vasogenic edema (Fig. 1A).
She underwent craniotomy for gross total resection of the lesion followed by stereotactic radiotherapy (SRT) to the cavity (Cyberknife, 3 fractions × 8 Gy). Tumor tissue obtained from the brain favored metastatic, poorly differentiated carcinoma suggestive of gastrointestinal origin, possibly appendiceal. Blood biomarker showed a modestly elevated carcinoembryonic antigen (CEA) of 8.5 ng/mL and CA125 of 59 U/mL. To identify primary origin, she underwent an esophagogastroduodenoscopy and colonoscopy. A 1-cm mass with stigmata of recent bleeding was identified within the appendiceal orifice and sampled, confirming primary appendiceal carcinoma (Fig. 1B). On the basis of her age, right-sided primary lesion location, and loss of mismatch repair proteins, she was suspected to have had Lynch syndrome but germline molecular testing was not performed. She initiated palliative FOLFOX (oxaliplatin, 5-fluorouracil, and leucovorin) chemotherapy, transitioning after two cycles to pembrolizumab due to hepatic progression. In July 2019, SRT was repeated for 3 new metastatic foci within the brain; in August 2019, new lesions in the right caudate and medulla oblongata were observed. In September 2019, she was readmitted to the hospital for disease progression. She forewent further treatment and passed away within the month.
A section of red-tan soft tissue was obtained from the right frontal lobe mass. Histopathology revealed a poorly differentiated neoplasm with prominent sheet-like growth, prominent nucleoli and mitoses, and necrosis (Fig. 2). The lesion was positive for cytokeratin cocktail (AE1/AE3, Cam5.2), CK7 (focal), CDX-2, p63 (focal), CK5/6 (focal), glutamine synthetase (patchy), CD10, and polyclonal CEA (cytoplasmic) staining. There was negative staining for CK20, PAX8, WT1, GATA3, TTF1, napsin, p40, mammoglobin, synaptophysin, glypican 3, GFAP, Oct3/4, and SALL4. HepPar1 staining was equivocal. FoundationOne molecular profiling demonstrated biomarkers of high microsatellite instability and intermediate tumor mutational burden (13 Muts/Mb) as well as genomic mutations of ARID1A, CDK12, CTNNB1, PIK3R1, PTEN, SETD2, and SOX9. Biopsy from the appendiceal mass revealed a poorly differentiated neoplasm with prominent sheet-like growth and mitotic activity morphologically consistent with the brain tumor resection. Areas of glandular differentiation appeared predominantly reactive and representative of non-neoplastic tissue entrapped within the biopsy specimen (Fig. 3). Immunohistochemistry for DNA mismatch repair proteins showed loss of staining for MLH1 and PMS2 in the tumor cells, with intact staining of MSH2 and MSH6. On the basis of morphology, coloss of MLH1 and PMS2, and microsatellite instability with an ARID1A mutation, a primary diagnosis of medullary carcinoma (MC) of the appendix was favored.
We describe a widely metastatic appendiceal cancer in a 47-year-old female who presented with a symptomatic cerebral metastasis following one year of vague gastrointestinal complaints. Appendiceal cancer is rarely suspected before diagnosis because its symptoms are often non-specific, such as discomfort, bloating, or weight changes, or it is incidentally discovered on surgical pathology specimens following appendectomy for appendicitis. Consequently, its diagnosis is often delayed [2]. In a SEER database analysis, Minhas et al. [2] estimated that 38.4% of appendiceal adenocarcinomas present with metastatic spread with an associated with a median survival of 35 months. The peritoneum and regional lymph nodes are the most common metastatic sites followed by liver and lung [2]. Management of the primary site entails right hemicolectomy, which has demonstrated survival benefit over appendectomy. Drawing on colorectal cancer treatment paradigms, the presence of nodal involvement supports using 5-FU-based systemic and intraperitoneal chemotherapy regimens [7].
The 2019 World Health Organization (WHO) classifies primary appendiceal cancers into four main categories: nonadenocarcinoma mucinous neoplasms, adenocarcinomas (signet-ring, mucinous, nonmucinous/colorectal-type), goblet cell, and neuroendocrine (carcinoid) [8]. Rarer appendiceal primaries also exist, including non-Hodgkin’s lymphomas and ganglioneuromas [7]. The average age at diagnosis of a noncarcinoid primary appendiceal cancer is 55–65 years, whereas carcinoids are typically diagnosed late in the fourth decade of life [7]. Histologic subtype and TNM system staging are the crucial factors affecting overall 5-year survival with carcinoids faring best and signet ring faring worst (18% 5-year survival) [27].
A literature search of PubMed, Embase, and Google Scholar identified six additional cases of cerebral appendiceal metastases (Fig. 4 and Table 1) [569101112]. The primary tumor histology of cerebral appendiceal metastases was mucinous adenocarcinoma in 3 cases and non-mucinous adenocarcinoma, goblet cell, and unspecified adenocarcinoma each in 1 case. The age at their primary diagnosis ranged from 33–72 years (average 55.8±16.0). Hemi-colectomy was performed in 5/6 cases that developed cerebral appendiceal metastases with our case proceeding immediately to FOLFOX chemotherapy in light of the systemic disease burden at presentation.
Performing a systematic review of brain metastases in colorectal cancer, Christensen et al. [13] reported that the median interval from primary diagnosis to brain metastasis ranged from 20–40 months. They reported that 87.7% of patients with brain metastases develop extracranial metastases and the interval from a diagnosis of an extracranial metastasis to brain metastasis is 9–23 months. Prognosis following brain metastasis is grim and ranges from 1 to 13 months [3]. Jung et al. [14] reported on 126 patients with cerebral colorectal metastases at their institution and observed a median survival of only 5.4 months after diagnosis and 1-year survival of 11.5%. On univariate analysis, they reported improved median survival with radiosurgery (9.5 months) and surgical resection (11.5 months) compared to supportive care (1.5 months) and whole brain radiation (4.0 months) but also noted that solitary lesions fared better than multiple lesions (9.0 months vs. 4.0–4.7 months) [14]. We note that all 7 cases of cerebral appendiceal metastases had extracranial metastases at intracranial diagnosis and in 2/4 cases the diagnosis of an appendiceal brain metastasis was made at the same time as part of the patient’s initial presentation. Management of the cerebral metastasis was described in 4 cases, and in 3 cases gross total resection and radiation were utilized. Survival appeared poor as 5/7 cases were dead at final follow-up; however, only 4 cases reported follow-up length after intracranial metastasis (range 2–7 months).
MC is an uncommon gastrointestinal histopathology primarily associated with colorectal cancer. It is characterized by a syncytial growth pattern, vesicular nuclei with conspicuous nucleoli, marked eosinophilic cytoplasm, and numerous intraepithelial lymphocytes. As a poorly differentiated carcinoma, it can be difficult to discriminate from poorly differentiated adenocarcinoma (PDA). Although the WHO define MCs as lacking features of glandular or intestinal differentiation, most studies allow for some evidence of differentiation [15]. The criteria suggested by the American Joint Committee on Cancer/College of American Pathologists recommendations for a MC diagnosis is ≥50% of the tumor lacking overt glandular formation. Study variation in the degree of glandular differentiation allowed has led to a range of prevalence estimates for MC (0.1%–2.8% of colorectal carcinomas) [16].
Immunohistochemical and molecular markers proposed to differentiate MC from PDA include expression of calretinin [17], loss MLH-1 [1517], loss of PMS-2 [15], loss of CDX2 [17], loss of p53 [15], BRAF mutations (among patients without Lynch syndrome) [18], and ARID1A mutations [19]. Winn et al. [17] reported a positive predictive value of 82% for MC when there is calretinin expression, MLH-1 loss, and CDX2-loss. However, only microsatellite instability and loss of MLH-1 have been reliably reproduced across studies as significantly more common in MC than PDA, and no single feature fully determines diagnosis [1517]. As such, some authors would prefer classifying poorly differentiated carcinomas by molecular analyses and microsatellite status rather than histopathology [15]. Although prognosis in MC has been previously felt to be more favorable than PDA [1718], survival benefit is markedly reduced when controlling for mismatch repair protein deficiency [16] or ARID1A mutations [19]. With the understanding that our case would represent the first description of primary MC arising within the appendix, we favored a diagnosis of MC. Because calretinin staining was not performed and the primary tissue analyzed was a biopsy specimen, the diagnosis of MC was rendered as “probable.”
Primary appendiceal carcinoma metastatic to the brain is an extremely rare phenomenon. We performed the first comprehensive literature review for cerebral appendiceal metastases and describe the seventh case. Clinicians should be aware that cerebral appendiceal metastases typically also present with extracranial metastases and portend poor prognosis with 5/7 cases dead at final follow-up. The most common primary appendiceal histology to the brain is mucinous adenocarcinoma, which was reported in 3/7 cases. Our case is further significant for having illustrated the first histologic diagnosis of primary MC in the appendix and observed its capacity to metastasize to brain.
Notes
Ethics Statement: This report was conducted according to the guidelines of the Declaration of Helsinki for biomedical research, and was deemed exempt from patient consent by the Institutional Review Board (2021P000600).
Availability of Data and Material
The datasets generated or analyzed during the study may be available from the corresponding author on reasonable request.
References
1. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020; 70:145–164. PMID: 32133645.
2. Minhas A, Hendrickson J, Minhas SA. Frequency and risk factors for metastasis in newly diagnosed appendiceal carcinoma. Cureus. 2021; 13:e16341. PMID: 34395124.
3. Thompson E, Banerjee S, Thompson S, Silva R, Muse A, Arif-Tiwari H, et al. Incidence and predictors of brain metastasis in colorectal cancer patients. Int J Colorectal Dis. 2022; 37:153–159. PMID: 34596736.
4. Habbous S, Forster K, Darling G, Jerzak K, Holloway CMB, Sahgal A, et al. Incidence and real-world burden of brain metastases from solid tumors and hematologic malignancies in Ontario: a population-based study. Neurooncol Adv. 2021; 3:vdaa178. PMID: 33585818.
5. Biroli A, Cecchi PC, Pragal S, Hanspeter E, Schwarz A. Cerebral metastasis from a previously undiagnosed appendiceal adenocarcinoma. Case Rep Oncol Med. 2012; 2012:192807. PMID: 23198200.
6. Foster A, Lofters J, Durham S, Jhawer M. A rare case of brain metastases from appendiceal carcinoma: a case report. J Case Rep Images Oncology. 2021; 7:100080Z10AF2021.
7. Ruoff C, Hanna L, Zhi W, Shahzad G, Gotlieb V, Saif MW. Cancers of the appendix: review of the literatures. ISRN Oncol. 2011; 2011:728579. PMID: 22084738.
8. Washington MK, Nagtegaal ID. Tumours of the appendix: Introduction. WHO Classification of Tumours Editorial Board. WHO classification of tumours. Digestive system tumours. 5th ed. Lyon: IARC Press;2019. p. 135–156.
9. Roy SP, Al Zhahrani N, Barat S, Morris DL. Case series on high grade appendiceal cancer with peritoneal and liver carcinomatosis undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Int J Surg Case Rep. 2022; 94:107027. PMID: 35398783.
10. Nonaka D, Papaxoinis G, Lamarca A, Fulford P, Valle J, Chakrabarty B. A study of appendiceal crypt cell adenocarcinoma (so-called goblet cell carcinoid and its related adenocarcinoma). Hum Pathol. 2018; 72:18–27. PMID: 28823572.
11. Dietrich CS 3rd, Desimone CP, Modesitt SC, Depriest PD, Ueland FR, Pavlik EJ, et al. Primary appendiceal cancer: gynecologic manifestations and treatment options. Gynecol Oncol. 2007; 104:602–606. PMID: 17055559.
12. Spann JL, Lowbeer L, VanWormer DE. Adenocarcinoma of the appendix vermiformis. J Surg Oncol. 1971; 3:185–196. PMID: 5094277.
13. Christensen TD, Spindler KL, Palshof JA, Nielsen DL. Systematic review: brain metastases from colorectal cancer—incidence and patient characteristics. BMC Cancer. 2016; 16:260. PMID: 27037031.
14. Jung M, Ahn JB, Chang JH, Suh CO, Hong S, Roh JK, et al. Brain metastases from colorectal carcinoma: prognostic factors and outcome. J Neurooncol. 2011; 101:49–55. PMID: 20467783.
15. Fiehn AM, Grauslund M, Glenthøj A, Melchior LC, Vainer B, Willemoe GL. Medullary carcinoma of the colon: can the undifferentiated be differentiated? Virchows Arch. 2015; 466:13–20. PMID: 25339302.
16. Lee LH, Yantiss RK, Sadot E, Ren B, Calvacanti MS, Hechtman JF, et al. Diagnosing colorectal medullary carcinoma: interobserver variability and clinicopathological implications. Hum Pathol. 2017; 62:74–82. PMID: 28034727.
17. Winn B, Tavares R, Fanion J, Noble L, Gao J, Sabo E, et al. Differentiating the undifferentiated: immunohistochemical profile of medullary carcinoma of the colon with an emphasis on intestinal differentiation. Hum Pathol. 2009; 40:398–404. PMID: 18992917.
18. Knox RD, Luey N, Sioson L, Kedziora A, Clarkson A, Watson N, et al. Medullary colorectal carcinoma revisited: a clinical and pathological study of 102 cases. Ann Surg Oncol. 2015; 22:2988–2996. PMID: 25572685.
19. Ye J, Zhou Y, Weiser MR, Gönen M, Zhang L, Samdani T, et al. Immunohistochemical detection of ARID1A in colorectal carcinoma: loss of staining is associated with sporadic microsatellite unstable tumors with medullary histology and high TNM stage. Hum Pathol. 2014; 45:2430–2436. PMID: 25311944.
Fig. 1
Images of appendiceal carcinoma with cerebral metastasis. A: Axial T1-gadolinum enhanced MRI of the brain demonstrating a heterogeneously enhancing right frontal mass with extensive vasogenic edema prior to resection. B: Appendiceal mass identified on colonoscopy with stigmata of recent bleeding.
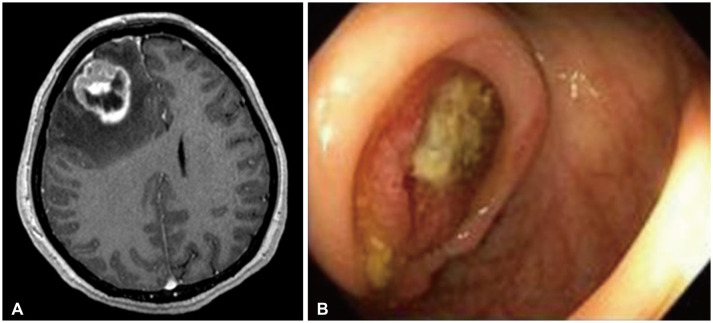
Fig. 2
Photomicrographs of the right frontal mass showing metastatic poorly differentiated carcinoma. A: Low power (H&E, ×4) shows a poorly differentiated neoplasm with prominent sheet-like growth and necrosis (bottom). B: Higher power (H&E, ×20) highlights poorly differentiated tumor cells with prominent nucleoli and mitotic activity. C: CDX-2 is positive in tumor cells, suggesting gastrointestinal origin. Scale bars = 200 µm (A), 50 µm (B), 100 µm (C).
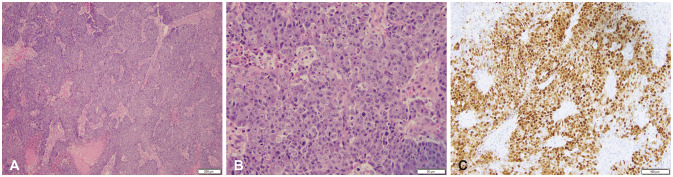
Fig. 3
Photomicrographs of the appendix tumor biopsy, poor differentiated carcinoma, favoring medullary carcinoma. A: High power (H&E, ×20) highlights a poorly differentiated tumor with sheet-like growth, prominent nucleoli, and mitotic activity, similar to the brain lesion. B: Immunohistochemistry for mismatch repair protein MLH-1 shows loss in tumor cells with retention in adjacent non-neoplastic cells. C: Immunohistochemistry for mismatch repair protein PMS-2 shows loss in tumor cells with retention in adjacent non-neoplastic cells. Scale bars = 50 µm (A), 100 µm (B and C).
Fig. 4
Flow chart of search results for cerebral appendiceal metastases performed May 2022 using the databases of PubMed, Embase, and Google Scholar.
Table 1
Literature review of clinical characteristics of patients with cerebral appendiceal cancer metastases
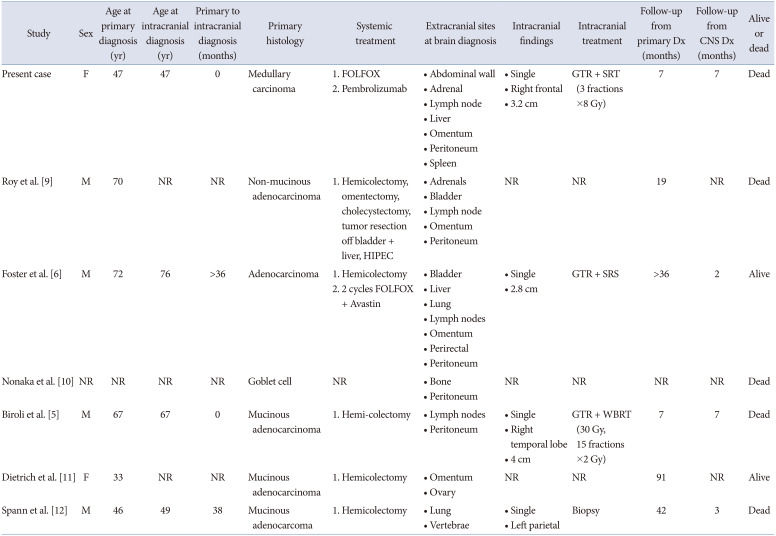
| Study | Sex | Age at primary diagnosis (yr) | Age at intracranial diagnosis (yr) | Primary to intracranial diagnosis (months) | Primary histology | Systemic treatment | Extracranial sites at brain diagnosis | Intracranial findings | Intracranial treatment | Follow-up from primary Dx (months) | Follow-up from CNS Dx (months) | Alive or dead |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present case | F | 47 | 47 | 0 | Medullary carcinoma |
1. FOLFOX 2. Pembrolizumab |
• Abdominal wall • Adrenal • Lymph node • Liver • Omentum • Peritoneum • Spleen |
• Single • Right frontal • 3.2 cm |
GTR + SRT (3 fractions ×8 Gy) | 7 | 7 | Dead |
| Roy et al. [9] | M | 70 | NR | NR | Non-mucinous adenocarcinoma | 1. Hemicolectomy, omentectomy, cholecystectomy, tumor resection off bladder + liver, HIPEC |
• Adrenals • Bladder • Lymph node • Omentum • Peritoneum |
NR | NR | 19 | NR | Dead |
| Foster et al. [6] | M | 72 | 76 | >36 | Adenocarcinoma |
1. Hemicolectomy 2. 2 cycles FOLFOX + Avastin |
• Bladder • Liver • Lung • Lymph nodes • Omentum • Perirectal • Peritoneum |
• Single • 2.8 cm |
GTR + SRS | >36 | 2 | Alive |
| Nonaka et al. [10] | NR | NR | NR | NR | Goblet cell | NR |
• Bone • Peritoneum |
NR | NR | NR | NR | Dead |
| Biroli et al. [5] | M | 67 | 67 | 0 | Mucinous adenocarcinoma | 1. Hemi-colectomy |
• Lymph nodes • Peritoneum |
• Single • Right temporal lobe • 4 cm |
GTR + WBRT (30 Gy, 15 fractions ×2 Gy) | 7 | 7 | Dead |
| Dietrich et al. [11] | F | 33 | NR | NR | Mucinous adenocarcinoma | 1. Hemicolectomy |
• Omentum • Ovary |
NR | NR | 91 | NR | Alive |
| Spann et al. [12] | M | 46 | 49 | 38 | Mucinous adenocarcoma | 1. Hemicolectomy |
• Lung • Vertebrae |
• Single • Left parietal |
Biopsy | 42 | 3 | Dead |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download