Abstract
Radiation-induced cavernous hemangiomas (RICHs) have been increasingly reported as a late complication after conventional radiotherapy. RICH after stereotactic radiosurgery (SRS) is extremely rare and the few cases have been reported to demonstrate their properties. A 72-year-old female patient presented with progressive neurologic deficits. She underwent tumor surgery for meningioma 13 years ago and two times of SRS for treating a residual tumor. Newly-developed mass was 4.3 cm-sized heterogeneously enhancing mass with severe cerebral edema. She underwent surgical resection and the histologic examinations revealed organized hematoma. Finally, it was diagnosed as a RICH following SRS based on radiological and histological findings and a history of multiple radiosurgeries. Clinical, radiological, and histological features of a RICH following SRS were discussed in this report.
Stereotactic radiosurgery (SRS) is an established treatment modality to treat intracranial meningioma. Radiation-induced neoplasm is widely accepted as a risk of radiation exposures [1]. Radiation-induced cavernous hemangiomas (RICHs) have been increasingly reported as a late complication after conventional radiotherapy (RT) [2]. Similar to de novo sporadic cavernous malformation, RICH is a popcorn-like enhancing lesion with a hemosiderin rim on MRI, and is a vascular-rich hemorrhagic lesion on histologic examination [3]. Several recent studies have investigated the clinical characteristics, hemorrhagic risk, and histologic features of the RICH after RT [45]. However, RICHs after SRS are rare and the few cases have been reported to demonstrate their properties. Here, we report a case of huge RICH, which was developed from a partially resected meningioma after repeated treatment of SRS.
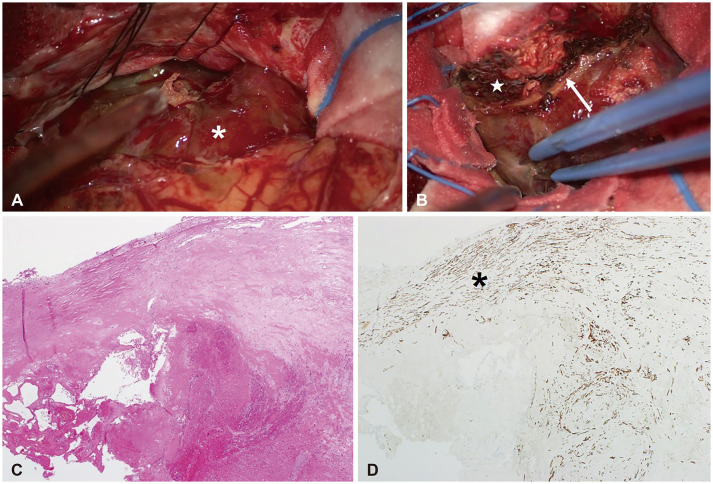
A 72-year-old female patient presented with headache, aphasia, and a progressive cognitive decline. She underwent craniotomy for a sphenoid ridge meningioma 13 years ago in other hospital. The initial tumor was 6 cm-sized hypervascular mass with severe adhesion to the middle cerebral artery, and was removed partially. Histological examination revealed fibrous meningioma. At six months after surgery, residual mass was treated with Gamma Knife (Elekta Instruments, Stockholm, Sweden) radiosurgery (GKS). The tumor volume was 12.3 mL and the prescription dose was 13 Gy at 50% isodose line (Fig. 1). The tumor was shrunk and stable until 3 years after GKS except a 2 cm-sized nodular protruded lesion which was gradually enlarged. The secondary re-irradiation using GKS for the nodular lesion was delivered at 3 years after the first GKS with 15 Gy at 50% isodose line. The lesion was successfully regressed and was stable thereafter until the last follow-up MRI which was performed one year before the emergent visit with neurologic symptom. In our clinic, MRI was performed and revealed a 4.3 cm-sized heterogeneously enhancing mass with salt and pepper appearance. The lesion was accompanied by severe surrounding cerebral edema. Radiologic findings of partial hemosiderin rim and cystic degeneration were also noted (Fig. 2). The time interval was 12.5 years from the first GKS, and was 9.5 years from the second GKS. In this case, a thrombosed aneurysm or acquired vascular malformations should be evaluated. Therefore, cerebral angiography was performed and no vascular connection with the mass (radiological occult lesion) was observed. She underwent microsurgery through a same surgical corridor used in the initial surgery. During surgery, the lesion appeared purplish-red and was well-defined. The capsule of lesion was thick, elastic, and highly-vascularized. The inner portion of lesion was filled with organized hematoma, calcification, and granulation tissue without viable component (Fig. 3). The lesion was completely removed and the patient’s symptoms were immediately relieved. The histologic examinations revealed organized hematoma with calcification and acellular materials, and no tumor tissue. Smooth muscle actin immunohistochemical staining revealed muscle bundles in the thick capsule layer. Finally, it was concluded that the lesion was a RICH based on radiological and histological findings and a history of multiple radiosurgeries. Six months after surgery, the patient remained stable without any neurologic deficit.
RICH has been considered a sporadic form of cavernous hemangioma as a late complication of brain irradiation. RICH mainly occurs in patients who underwent cranial irradiation during childhood for the treatment of hematologic malignancies such as acute lymphoblastic leukemia, medulloblastoma, glioma, or arteriovenous malformation [2]. The incidence of RICH after RT increases with time 3%–4% at 10 years, 7%–14% at 20 years, and approximately 60% at 25 years [67]. The median time to detect RICH after RT is 8–12 years, and the latency appears to be shorter in the patients receiving RT in older age [489]. Several studies indicated that the younger the patient at the time of RT was associated with higher likelihood of multiple RICH but was not predictive of presence of symptoms or risk of hemorrhage. RICHs in cortical locations were less likely to be symptomatic at the time of diagnosis and had a lower risk of hemorrhage [410]. Keezer et al. [9], who gathered the previously reported RICH cases (n=84), argued that radiation doses and symptomatic presentation including hemorrhage of RICHs may have an inverse relationship. In adults, a few RICHs after RT have been reported, and the chance for their development seems to be dose dependent [6810]. The majority of RICHs have been asymptomatic and the most common presenting symptom is seizure in symptomatic patients [2]. Several studies recommend watchful waiting for asymptomatic RICH, reserving surgical resection for symptomatic growth or hemorrhagic transformation [211].
SRS is now widely used to treat a variety of brain lesions including brain tumors and vascular malformations. However, high-dose radiation possibly induces cerebral edema, radionecrosis, reactive gliosis, and telangiectasia, as the early and late radiation-induced effects. SRS emits a high dose of radiation to a small target area, leading to the shrinkage of the target lesion, which in turn results in a small cavitary lesion in the center of the focal area. Some authors hypothesized that RICH may originate not from radiation-induced vascular malformation, but the cavitary lesion due to the focal high-dose radiation resulting in tumor shrinkage [3]. This cavitary lesion would be filled with blood and local inflammatory materials, and a stabilized hematoma would be made gradually. The proliferation of thin-walled flawed vasculature and macrophage aggregation in RICHs are likely to be the radiation-related changes, rather than the findings of de novo carvernous hemangioma. Furthermore, most RICHs shows mixed intensity with an enhancing cystic and/or solid component and an incomplete hemosiderin rim on MRI, which is different from the findings of de novo carvernous hemangioma.
Several authors reported RICH after SRS treating various pathologies including metastatic brain tumor, pinealocytoma, arteriovenous malformation and vestibular schwannoma [12131415]. Nagy et al. [8] observed 3 RICHs in 425 patients (0.7%) with benign meningioma treated with GKS [8]. They analyzed an estimate of risk of developing a RICH after SRS was 0.2% at 5 years and 0.9% at 15 years. The radiation dose at the site of RICH formation shows no trend (6, 10, and 18 Gy) although marginal doses for the original lesions were not reported. In their study, two patients were asymptomatic and treated conservatively, and other two patients were symptomatic. Those two asymptomatic lesions increased in size during the follow-up period. In our patient, the cumulative dose of two irradiations at the margin of original meningioma was estimated to be 28 Gy. This dose was relatively higher than those used in other reports. The locations of RICHs after SRS reported so far, including our case, were identical or close to the sites of SRS, where original lesions existed [3481215]. In addition, most RICHs following SRS increased in size and/or caused significant cerebral edema. We therefore carefully consider that post-SRS RICHs originating the field of high-dose irradiation, i.e., RICHs located in the same locations as the original tumor rather than normal brain parenchyma, should be classified differently from ordinary RICHs. This hypothesis should be investigated in the further studies.
In our presenting case, the tumor treated with GKS was well controlled until one year before the symptom presentation and showed no portent to sprout the RICH. RICH in our patient is relatively huge and shows the potency that it can grow rapidly and accompany with a progressive neurologic symptoms and signs, as well as severe cerebral edema. Surgical resection is mandatory for a huge, rapid-growing, and symptomatic RICH to relieve mass effect and to verify histology. Clinicians should consider the possibility of developing this rare pathology during follow-up period after SRS, especially after multiple SRS.
Notes
Ethics Statement: The institutional review board (IRB) exempted informed consent due to its retrospective nature and minimal risk for harm to the patient, and this report was conducted according to the guidelines of the Declaration of Helsinki for biomedical research.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Paddick I, Cameron A, Dimitriadis A. Extracranial dose and the risk of radiation-induced malignancy after intracranial stereotactic radiosurgery: is it time to establish a therapeutic reference level? Acta Neurochir (Wien). 2021; 163:971–979. PMID: 33325003.
2. Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 2006; 21:e4.
3. Cha YJ, Nahm JH, Ko JE, Shin HJ, Chang JH, Cho NH, et al. Pathological evaluation of radiation-induced vascular lesions of the brain: distinct from de novo cavernous hemangioma. Yonsei Med J. 2015; 56:1714–1720. PMID: 26446658.
4. Cutsforth-Gregory JK, Lanzino G, Link MJ, Brown RD Jr, Flemming KD. Characterization of radiation-induced cavernous malformations and comparison with a nonradiation cavernous malformation cohort. J Neurosurg. 2015; 122:1214–1222. PMID: 25699412.
5. Kleinschmidt-DeMasters BK, Lillehei KO. Radiation-induced cerebral vascular “malformations” at biopsy. J Neuropathol Exp Neurol. 2016; 75:1081–1092. PMID: 27694117.
6. Koike T, Yanagimachi N, Ishiguro H, Yabe H, Yabe M, Morimoto T, et al. High incidence of radiation-induced cavernous hemangioma in long-term survivors who underwent hematopoietic stem cell transplantation with radiation therapy during childhood or adolescence. Biol Blood Marrow Transplant. 2012; 18:1090–1098. PMID: 22198541.
7. Vinchon M, Leblond P, Caron S, Delestret I, Baroncini M, Coche B. Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Childs Nerv Syst. 2011; 27:445–453. PMID: 21234575.
8. Nagy G, McCutcheon BA, Giannini C, Link MJ, Pollock BE. Radiation-induced cavernous malformations after single-fraction meningioma radiosurgery. Oper Neurosurg (Hagerstown). 2018; 15:207–212. PMID: 29281070.
9. Keezer MR, Del Maestro R. Radiation-induced cavernous hemangiomas: case report and literature review. Can J Neurol Sci. 2009; 36:303–310. PMID: 19534329.
10. Heckl S, Aschoff A, Kunze S. Radiation-induced cavernous hemangiomas of the brain: a late effect predominantly in children. Cancer. 2002; 94:3285–3291. PMID: 12115362.
11. Di Giannatale A, Morana G, Rossi A, Cama A, Bertoluzzo L, Barra S, et al. Natural history of cavernous malformations in children with brain tumors treated with radiotherapy and chemotherapy. J Neurooncol. 2014; 117:311–320. PMID: 24515423.
12. Iwai Y, Yamanaka K, Yoshimura M. Intracerebral cavernous malformation induced by radiosurgery. Case report. Neurol Med Chir (Tokyo). 2007; 47:171–173. PMID: 17457021.
13. Park YS, Kim SH, Chang JH, Chang JW, Park YG. Radiosurgery for radiosurgery-induced cavernous malformation. World Neurosurg. 2011; 75:94–98. PMID: 21492671.
14. Sasagawa Y, Akai T, Itou S, Iizuka H. Gamma knife radiosurgery-induced cavernous hemangioma: case report. Neurosurgery. 2009; 64:E1006–E1007. PMID: 19404123.
15. Wang X, Hui XH, Liu JP, Mao Q. Radiation-induced cavernous malformation at the site of arteriovenous malformation following gamma knife radiosurgery: case report. Clin Neurol Neurosurg. 2012; 114:1287–1289. PMID: 22456056.
Fig. 1
Radiologic images of residual meningioma after initial surgical resection at the time of stereotactic radiosurgery (A and B). At 3 years after initial stereotactic radiosurgery, the tumor is shrunk near-completely except a nodular protruded lesion near the cavernous sinus (C and D).

Fig. 2
Radiological findings of radiation-induced cavernous hemangioma (RICH). RICH shows a popcorn-like appearance, heterogeneous enhancement, and partial hemosiderin rim similar to the de novo cavernous hemangioma; furthermore, unilocular cystic areas with lobulated solid component and prominent perilesional edema are also features of RICH. A: Gadolinium-enhanced T1-weighted sequence. B: T2-weighted sequence.
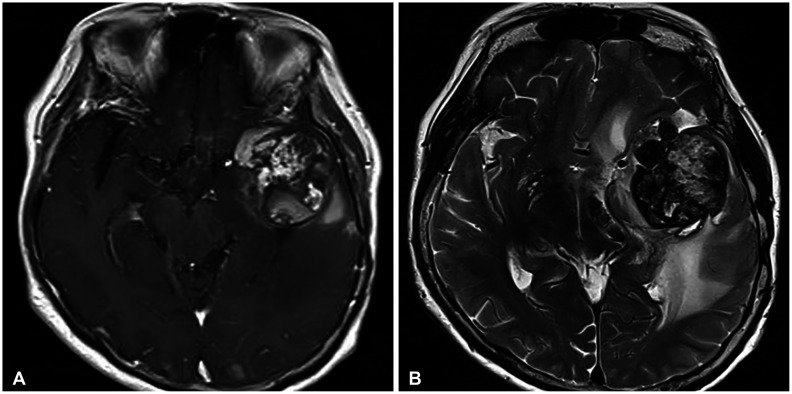
Fig. 3
Intraoperative and histologic findings of radiation-induced cavernous hemangioma (RICH) following radiosurgery. A: RICH occupying left middle cranial fossa shows a well-defined surface without grossly identifiable vascular structure (white asterisk). B: Thick capsule (white arrow) is highly vascularized and encapsulates organized hematoma (white star), calcification, and acellular granulation tissue. C: Microscopically, RICH shows hematoma-like area without viable tumor tissue and dilated thin-walled capillaries filled with thrombosed blood (H&E, ×40). D: Well-organized vasculature is not demonstrable in smooth muscle actin (SMA) immunohistochemical staining while the thick capsule is stained and delineated (black asterisk; SMA, ×40).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download