INTRODUCTION
Cerebral microangiopathy (CM), also known as cerebral small vessel disease, is being diagnosed with increasing frequency. The improvement in neuroimaging modalities, increased life expectancy, and dietary and life-style changes are possible reasons of increased diagnosis of CM [
1]. CM is one of the most prevalent pathological processes encountered by physicians [
23] and is characterized by pathological changes in small brain vessels, including small arteries, arterioles, capillaries, and veins [
4]. Some patients with CM can be asymptomatic; however, various related symptoms may also develop [
1]. Using brain MRI, CM can be diagnosed from the observance of the recent small subcortical infarcts, white matter hyperintensities, lacunes, cerebral microbleeds, cerebral atrophy [
5], and various radiological manifestations.
In the central nervous system (CNS), differentiation between tumors and tumor-like mass lesions is sometimes difficult, despite the use of advanced imaging techniques. Particularly when dealing with tumor-like lesions mimicking high-grade gliomas, physicians have to make an important decision regarding management, which can greatly impact the patient’s life. Generally, when a very elderly patient shows a high-grade glioma-like lesion on MRI, patients and caregivers hesitate to provide active treatment, including tissue confirmation. However, the recent European Association of Neuro-Oncology (EANO) guideline for CNS glioma indicates that a decision for palliative care without histologic diagnosis should be avoided unless the patient has severe comorbidities, or biopsy carries a high risk [
6]. Intra-axial mass lesions in elderly patients are more likely to be malignant tumors; however, a clinical decision based only on neuroimaging without tissue confirmation could be unhelpful to patients.
Here we present the case of a patient with CM mimicking a high-grade glioma on neuroimaging, whose caregivers hesitated to undergo treatment.
CASE REPORT
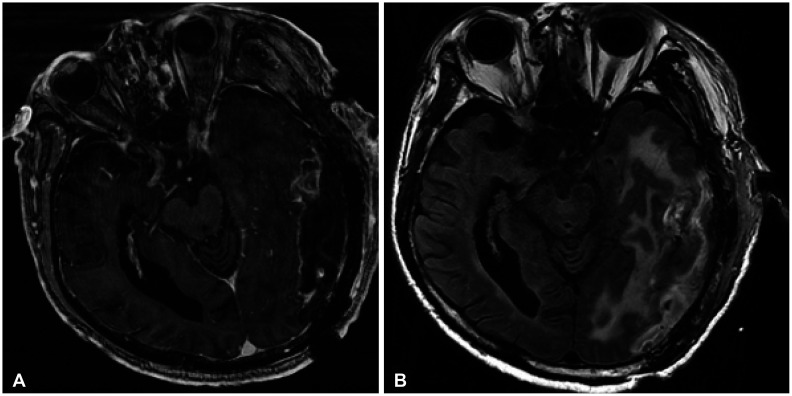
An 83-year-old man was admitted to our outpatient clinic with rapid progression of cognitive decline, gait disturbance, and voiding difficulty in the prior four weeks. The patient had no specific medical history of diseases, including hypertension and diabetes. There was no history of head trauma. No significant abnormal results were observed on physical examination or laboratory tests. The laboratory tests did not show dyslipidemia. His Korean Mini-Mental State Examination score was 7. Non-contrast brain CT showed a low-density, large mass lesion with partially ill-defined high density in the left posterior temporo-parietal area, which implied intratumoral hemorrhage (
Fig. 1A). Diffusion MRI revealed hemorrhagic mass in different phases (
Fig. 1B). Fluid attenuated inversion recovery images revealed severe peri-lesional edema and intra-axial cortical mass lesions along the left posterior temporo-parietal area (
Fig. 1C). This large nodular mass lesion showed contrast enhancement in the T-1 weighted contrast-enhancement images (
Fig. 1D and E). The gradient echo (GRE) images showed a partially low-signal on the intra-lesional hemorrhagic mass (
Fig. 1F).
Under the suspicion of high-grade glioma, the guardian argued against providing treatment because of the patient’s old age. However, the guardian and the patient agreed to confirm the pathology of the lesion. Tumor removal via craniotomy was performed. Intraoperatively, the arachnoid membrane was focally thickened with a whitish discoloration, and the cortical mass presented multiple small cortical hemorrhages (
Fig. 2A). The mass was reddish with high vascularity, and an intralesional hematoma was exposed (
Fig. 2B). The specimen was subjected to intraoperative assessment. However, contrary to our expectations, the frozen diagnosis of “no definite glial tumor” was rendered (
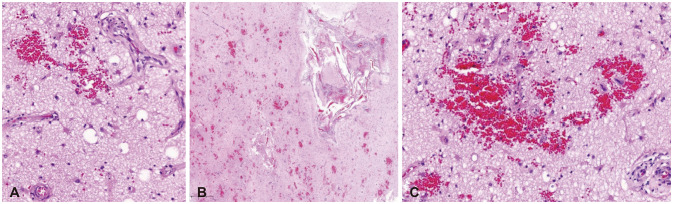
Fig. 3A). Pathologic examination revealed vascular proliferation with endothelial hyperplasia, slight thickening and fibrosis of the tunica media on the small sized arteries, reactive gliosis, and microscopic bleeding, which were multifocal and consistent with CM (
Fig. 3B and C). However, immunohistochemical stainings of amyloid A and Congo red did not show any accumulation. The postoperative image showed that the mass had been removed and that the perilesional edema had improved (
Fig. 4). The patient’s symptoms partly improved, but persisted because of his old age. The patient did not require any adjuvant treatment related to the high-grade glioma.
DISCUSSION
CM accounts for 25% of strokes [
7] and 45% of dementia cases [
8]. Its prevalence increases with age, affecting approximately 5% of people aged 50 years, to almost 100% of people older than 90 years [
9]. Arterial hypertension is the most significant modifiable risk factor [
10]. Clinical symptoms can vary, including seizures, vertigo, gait apraxia, urinary incontinence, stroke-related symptoms, progressive memory impairment, and dementia. On brain MRI, CM presents as a small subcortical infarct, white matter hyperintensities, cerebral microbleeds, lacunes, and cerebral atrophy [
11]. More rarely, CM can also be seen as a tumorous lesion with edema, as observed in our patient.
The pathogenesis of CM, although secondary to uncontrolled hypertension, includes hyaline and hyperplastic arteriolosclerosis, segmental arterial disorganization, and microaneurysm [
4]. Although poorly understood, the decreased cerebral blood flow leads to dysfunction of the neurovascular unit (NVU), which consists of neurons, astrocytes, endothelial cells, pericytes, and vascular smooth muscle cells [
12]; blood brain barrier (BBB) leakage, inflammation, edema, and oligodendrocyte dysfunction may occur secondary to hypoxia [
131415]. Severe ischemia of the small-vessel territory results in a small subcortical infarction, while leakage of blood products from a microaneurysm results in cerebral microbleeds [
5]. Although there was no disease history related to risk factors of atherosclerosis in our patient, the slight thickening and fibrosis of the tunica media and endothelial hyperplasia are shown on small-sized arteries in pathological results. These features may be related to artheriolosclerosis resulting in luminal narrowing and loss of elasticity [
16].
However, cerebral amyloid angiopathy (CAA) has a different pathogenesis from that of CM. CAA primarily affects the small- and medium-sized cortical and leptomeningeal arterioles and arteries. Amyloid-β peptide is deposited in the walls of these vessels, which causes loss of vessel compliance, decreased cerebral vascular reactivity, and increased susceptibility to cerebral microbleeds, cortical superficial siderosis, and cortical atrophy [
17]. In some previous studies, CAA rarely presented as a tumorous lesion, which is related to transmural and perivascular inflammation of amyloid deposits in small- and medium-sized vessels [
1819]. However, CM without amyloid angiopathy, which can be shown as a tumorous lesion, is more rarely presented, and our patient is a good example.
The most common imaging features of CAA are cortical and lobar macro- and micro-hemorrhages [
19]. Conversely, the radiologic characteristics of CM include recent small subcortical infarcts, white matter hyperintensities, lacunes, cerebral microbleeds, enlarged perivascular spaces, and cerebral atrophy [
5]. CAA related to tumorous lesions is known to result from inflammation of amyloid deposits, and its most common radiologic feature is the absence of parenchymal enhancement and, in a few cases, leptomeningeal enhancement. Therefore, due to the absence of parenchymal enhancement, the tumorous lesions related to CAA must be differentiated from low-grade gliomas, and GRE and susceptibility weighted imaging should be performed [
1819]. However, in our patient with CM, there was definite parenchymal enhancement. The parenchymal enhancement in T-1 weighted contrast-enhancement images could be shown in the late stage of acute cerebral infarction with hemorrhagic transformation. However, there was not predominantly high signal intensity in diffusion MRI. Therefore, this lesion is more likely to be radiologically diagnosed as high-grade glioma. We suppose that the possible reason for parenchymal enhancement is dysfunction of the NVU related to the dysfunction of small vessels, such as arterioles. This causes leakage of the BBB, which can be shown as contrast enhancement.
Hemorrhagic mass lesions with edema in very elderly patients have unfavorable outcomes, and most commonly result as secondary damage due to perihematomal injury through the induction of cerebral edema, which may worsen the clinical symptoms [
20]. However, unlike high-grade glioma, proper management, including the removal of a hemorrhagic mass, can improve patient prognosis. As recommended by the EANO guidelines for CNS glioma [
6], palliative care without histologic confirmation from radiological imaging alone can negatively affect our patient.
This case had one important limitation; magnetic resonance spectroscopy was not performed. Silek and Erbas [
18] described that magnetic resonance spectroscopy can be helpful in distinguishing angiopathies from tumors, in which CAA shows a normal N-acetylaspartate to creatinine (NAA/Cr) ratio and no increase in the cerebral choline/creatinine (Cho/Cr) ratio, revealing the normal metabolic state of the underlying brain tissue. However, our patient showed definite parenchymal enhancement and perilesional edema; therefore, we did not perform magnetic resonance spectroscopy.
In summary, we present the case of an elderly patient with CM mimicking a high-grade glioma on neuroimaging. Although the prevalence of CM is increasing, our patient presented a rare form of the CM. Herein, we compare the presentation of this case with that of CAA-mimicking tumorous lesions, which have different neuroimaging features.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download