Abstract
Approximately two-thirds of glioblastoma (GBM) patients progress to leptomeningeal spread (LMS) within two years. While 90% of LMS cases are diagnosed during the progression and/or recurrence of GBM (defined as secondary LMS), LMS presentation at the time of GBM diagnosis (defined as primary LMS) is very rare. 18F-fluorodeoxy glucose positron emission tomography computed tomography (18F-FDG PET/CT) study helps to diagnose the multifocal spread of the malignant primary brain tumor. Our patient was a 31-year-old man with a tumorous lesion located in the right temporal lobe, a wide area of the leptomeninges, and spinal cord (thoracic 5/6, and lumbar 1 level) involvement as a concurrent manifestation. After the removal of the right temporal tumor, the clinical status progressed rapidly, showing signs of increased intracranial pressure and hydrocephalus caused by LMS. He underwent a ventriculoperitoneal shunt a week after craniotomy. During management, progression of cord compression, paraplegia, bone marrow suppression related to radiochemotherapy, intercurrent infections, and persistent ascites due to peritoneal metastasis of the LMS through the shunt system was observed. The patient finally succumbed to the disease nine months after the diagnosis of simultaneous GBM and LMS. The overall survival of primary LMS with GBM in our case was nine months, which is shorter than that of secondary LMS with GBM. The survival period after the diagnosis of LMS did not seem to be significantly different between primary and secondary LMS. To determine the prognostic effect and difference between primary and secondary LMS, further cooperative studies with large-volume data analysis are warranted.
Glioblastoma (GBM) leptomeningeal spread (LMS) results from the spread of tumor cells from the brain parenchyma to the leptomeninges and cerebrospinal fluid (CSF) space and is considered one of the most severe complications of GBM [123].
The reported incidence of LMS after the diagnosis of GBM is approximately two-thirds of patients within the first two years of diagnosis. However, the estimated incidence of symptomatic LMS has been reported to be 2%. This is probably underestimated because of the undiagnosed and asymptomatic cases [1].
Since there is no standard treatment guideline for LMS in patients with GBM, it is considered as end-stage complication of the disease. The post-LMS diagnosis survival time was 0.2–9.7 months with a mean of 4.7 months [145].
Several therapeutic approaches for LMS, including intrathecal chemotherapy, radiation therapy, and recently, molecular targeted therapy, have been attempted; however, survival after diagnosis of LMS in GBM is not lengthened for more than eight months [6789101112].
We present a case of 31-year-old man with GBM and LMS diagnosed by early enhanced MRI and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) studies, which can suggest primary LMS.
A 31-year-old man was transferred from Dankook University Medical Center at Cheonan, where he presented with seizures, headache, and incipient papilledema. However, his neurological examination were normal with no history of extracranial primary carcinoma. Brain MRI revealed a small mass lesion localized in the right temporal lobe with leptomeningeal enhancement on the ventral side of the brain stem. No peritumoral edema or restricted diffusion was observed (Fig. 1). Contrast-enhancing lesions in the right temporal lobe and leptomeningeal contrast enhancement on the ventral side of the brain stem were seen on MRI, in addition 18F-FDG PET/CT showed multifocal high metabolic uptake lesions in the brain and spinal cord. We suspected a primary brain tumor with the LMS at the time of GBM diagnosis, which is very rare even in malignant primary brain tumors.
However, metastasis from an extracranial source needs to be ruled out; therefore, brain metastasis workups were performed. There was no enhancing tumorous lesion on CT scans of the abdomen and chest. Whole-body 18F-FDG PET/CT was also performed, which revealed high metabolic uptake lesions at the left central sulcus, left Sylvian cistern, skull base, and spinal cord of T5/6, L1 spinal levels (Fig. 2). A subsequent spinal MRI showed enhanced lesions corresponding to the 18F-FDG PET/CT image. Optic fundus examination revealed papilledema.
Craniotomy was performed for accurate diagnosis and excision of the tumor (Fig. 3). During surgery, the cortical surface anatomy appeared normal and the mass was located in the deep proximal Sylvian fissure. The superficial cortical subarachnoid space appeared normal, and leptomeningeal thickening was not observed. The tumor was composed of gray-colored soft tissue with no cystic portion. Gross total removal was performed. Intraoperative frozen biopsy revealed the tumor as a high-grade malignant tumor, such as metastasis or lymphomatous tumor, and is unlikely to be a high-grade glial tumor.
Pathologic examination of the specimen showed as follows. A microscopic view of the hematoxylin and eosin (H&E) stain revealed nuclear pleomorphism, mitosis, microvascular proliferation, and necrosis (Fig. 4). Immunohistochemical staining was positive for glial fibrillary acidic proteins. The final pathologic report revealed GBM with isocitrate dehydrogenease (IDH)-wild type (Grade 4) and positive O6-methylguanine methyltransferase (MGMT) promoter methylation.
Therefore, concurrent chemoradiation therapy was initiated. However, five days after surgery, the patient fell into a stupor and was unable to obey verbal commands. A follow-up brain CT scan showed no definite changes or intracranial hemorrhage, but the electrolyte profile exhibited salt-wasting syndrome. Seven days after craniotomy, the patient presented with uncontrolled intracranial hypertension and a decreased heart rate. Under the diagnosis of increased intracranial pressure (ICP) and papilledema with acute hydrocephalus, a ventriculo-peritoneal (VP) shunt was performed. Although a small amount of CSF had spilled out of the dura, the ICP was still 54 cm H2O. The dura mater was bulged and tense upon palpation. One week after the VP shunt, the patient recovered his alert mental status but exhibited paraplegia, and MRI confirmed increased tumor size at L1 spinal lesion (from 2.3 cm to 2.8 cm).
One month after craniotomy, the patient underwent modified low-dose raditherapy at 39.6 Gy due to bone marrow suppression. Temozolomide treatment was postponed for three weeks due to multi-focal pneumonia and fever.
The patient experienced moderate upper right quadrant abdominal pain. Abdominal CT has shown no definite changes since the last study. Since the circumference of the patient’s abdomen continued to increase, suggesting a normal-functioning VP shunt, abdominopelvic CT and whole-body 18F-FDG PET were performed. Follow-up abdominopelvic CT showed increased ascites, peritoneal thickening, and nodular infiltration. In addition, 18F-FDG PET/CT showed lesions with high metabolic uptake in the abdomen and pelvic bone, which was considered abdominopelvic metastasis (spread) of the GBM (Fig. 5). Paracentesis for relieving the distended abdomen was performed seven times. Nonetheless, evidence of malignant cells was not found in any of the cytological studies of ascitic fluid. The clinical status progressively deteriorated.
Nine months after the diagnosis of GBM and LMS, the patient succumbed to the disease with multiorgan failure due to septic conditions.
We present a rare case of primary GBM with simultaneous LMS (brain and spinal cord, primary LMS), followed by abdominal metastasis via a VP shunt. Other case reports of primary GBM with concurrent LMS at the time of diagnosis are summarized in Table 1 [413141516171819202122232425]. The LMS results from the spreading of tumor cells from the brain parenchyma via the perivascular space to the subpial, leptomeninges, and CSF. It is one of the most severe complications of GBM and implies a more aggressive behavior and a worse prognosis. Patients with LMS may experience increased ICP, hydrocephalus, and cranial nerve palsies [226]. Intractable vomiting may occur in patients with fourth ventricular involvement, but the frequency of seizures does not seem to increase because of LMS [27]. Moreover, a recent review article reported that two-thirds of patients with GBM develop LMS within the first two years after the diagnosis of GBM, and the median delay to the diagnosis of LMS varies from 5 to 16.4 months. In addition, this delay may be shorter in specific tumor locations, such as the pineal, spinal, periventricular, and infratentorial regions [1228]. In this case, the LMS was present in the whole brain and spinal cord at the time of the diagnosis of temporal lobe GBM, which was in contact with the Sylvian cistern and may have contributed to the LMS. This pattern of LMS at the time of GBM diagnosis is very rare compared to secondary LMS during the treatment or recurrence of GBM.
No risk factors for LMS of GBM have been demonstrated, although multiple factors have been suggested, such as age, histological features, molecular alterations, anatomical tumor site, tumor volume, and therapeutic interventions [1].
In retrospective studies and case series regarding GBM with LMS, the mean overall survival after diagnosis of treated LMS was 4.94 months (range, 2–9 months). Our patient survived for 9 months after the diagnosis of LMS and GBM. However, the patient showed rapid initial disease progression. One week after tumor removal, the patient underwent a VP shunt due to increased ICP and hydrocephalus with a stupor mentality. One month after the tumor removal, the patient developed paraplegia.
The LMS of GBM can be classified into two types. The first type is a positive parenchymal GBM with concurrent LMS at diagnosis (primary LMS). The second type is GBM with delayed LMS during treatment or recurrence of GBM (secondary LMS). Most LMS cases of GBM are of the secondary LMS type. However, our case was of the first type (primary LMS).
A comparison between the survival time of our patient (primary LMS) and the current mean overall survival data for GBM (secondary LMS) indicated a shorter overall survival of our patient (9 months); however, if survival after diagnosis of LMS was compared, the survival time of our patient was in the upper range of the survival data (2–9 months). Primary and secondary LMS survival after the diagnosis of LMS did not seem to differ, which means that LMS negatively affected the overall prognosis of GBM.
As the patient’s abdominal circumference continued to increase, we had to do consecutive paracentesis seven times to relieve abdominal distension and alleviate compromised respiration. The aspiration fluid volume varied from 200 mL to 1,000 mL each time. All the samples were used for the fluid cytology test, which was negative for malignant cells. A direct peritoneal biopsy may be an option for the diagnosis of LMS in cases with repeated negative ascitic fluid analysis of malignant cells. However, in this case, a surgical peritoneal biopsy was not performed because of poor performance status and physical burden, a probable surgical complication related to a distended abdomen due to ascites, and no additional treatment modality could be beneficial to the patient. CSF cytologic positivity in GBM with LMS is variable, 4%–75% of cases [131329]. From a practical point of view, there is no doubt that persistent ascites despite repeated paracenteses are related to the peritoneal spread of GBM cells and compromising the peritoneal CSF absorption capacity, not the problem of shunt malfunction. In this case, 18F-FDG PET/CT was helpful for the clinical diagnosis of LMS in the peritoneal cavity.
In conclusion, the overall survival of primary LMS with GBM in our case was nine months, which is shorter than that of secondary LMS with GBM. The survival period after the diagnosis of LMS did not seem to be significantly different between primary and secondary LMS. To evaluate the prognostic effect and differences between primary and secondary LMS, further cooperative studies with large volumes of data are warranted.
Notes
Ethics Statement: The Armed Forces Capital Hospital IRB (IRB number: 2021-12-007-001) approved this study, and the patient’s consent form was waived.
Author Contributions:
Conceptualization: Cheolwon Jang, Byung-Kyu Cho.
Data curation: Cheolwon Jang, Sung Hwan Hwang, Byung-Kyu Cho.
Investigation: Cheolwon Jang, Byung-Kyu Cho, Hyung Jin Shin.
Supervision: Byung-Kyu Cho.
Visualization: Cheolwon Jang, Sung Hwan Hwang, Hyung Jin Shin, Sang Hoon Yoon.
Writing—original draft: Cheolwon Jang.
Writing—review & editing: Byung-Kyu Cho.
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available because of privacy and ethical concerns but are available from the corresponding author upon reasonable request.
References
1. Birzu C, Tran S, Bielle F, Touat M, Mokhtari K, Younan N, et al. Leptomeningeal spread in glioblastoma: diagnostic and therapeutic challenges. Oncologist. 2020; 25:e1763–e1776. PMID: 33394574.
2. Autran D, Barrie M, Matta M, Monserrat C, Campello C, Petrirena G, et al. Leptomeningeal gliomatosis: a single institution study of 31 patients. Anticancer Res. 2019; 39:1035–1041. PMID: 30711992.
3. Noh JH, Lee MH, Kim WS, Lim DH, Kim ST, Kong DS, et al. Optimal treatment of leptomeningeal spread in glioblastoma: analysis of risk factors and outcome. Acta Neurochir (Wien). 2015; 157:569–576. PMID: 25663100.
4. Mandel JJ, Yust-Katz S, Cachia D, Wu J, Liu D, de Groot JF, et al. Leptomeningeal dissemination in glioblastoma; an inspection of risk factors, treatment, and outcomes at a single institution. J Neurooncol. 2014; 120:597–605. PMID: 25168214.
5. Zhao KH, Zhang C, Bai Y, Li Y, Kang X, Chen JX, et al. Antiglioma effects of cytarabine on leptomeningeal metastasis of high-grade glioma by targeting the PI3K/Akt/mTOR pathway. Drug Des Devel Ther. 2017; 11:1905–1915.
6. Brown MT, Coleman RE, Friedman AH, Friedman HS, McLendon RE, Reiman R, et al. Intrathecal 131I-labeled antitenascin monoclonal antibody 81C6 treatment of patients with leptomeningeal neoplasms or primary brain tumor resection cavities with subarachnoid communication: phase I trial results. Clin Cancer Res. 1996; 2:963–972. PMID: 9816257.
7. Chamberlain MC. Combined-modality treatment of leptomeningeal gliomatosis. Neurosurgery. 2003; 52:324–329. discussion 330. PMID: 12535360.
8. Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999; 5:3394–3402. PMID: 10589750.
9. Leaver KE, Zhang N, Ziskin JL, Vogel H, Recht L, Thomas RP. Response of metastatic glioma to vemurafenib. Neurooncol Pract. 2016; 3:268–271. PMID: 31386052.
10. Burger MC, Zeiner PS, Jahnke K, Wagner M, Mittelbronn M, Steinbach JP. Addition of anti-angiogenetic therapy with bevacizumab to chemo- and radiotherapy for leptomeningeal metastases in primary brain tumors. PLoS One. 2016; 11:e0155315. PMID: 27253224.
11. Kanemaru Y, Natsumeda M, Okada M, Saito R, Kobayashi D, Eda T, et al. Dramatic response of BRAF V600E-mutant epithelioid glioblastoma to combination therapy with BRAF and MEK inhibitor: establishment and xenograft of a cell line to predict clinical efficacy. Acta Neuropathol Commun. 2019; 7:119. PMID: 31345255.
12. Woo PYM, Lam TC, Pu JKS, Li LF, Leung RCY, Ho JMK, et al. Regression of BRAFV600E mutant adult glioblastoma after primary combined BRAF-MEK inhibitor targeted therapy: a report of two cases. Oncotarget. 2019; 10:3818–3826. PMID: 31217909.
13. Andersen BM, Miranda C, Hatzoglou V, DeAngelis LM, Miller AM. Leptomeningeal metastases in glioma: The Memorial Sloan Kettering Cancer Center experience. Neurology. 2019; 92:e2483–e2491. PMID: 31019097.
14. Kwon JE, Hwang K, Go KO, Wee CW, Kim IA, Kim YJ, et al. Clinical characteristics of high-grade glioma with primary leptomeningeal seeding at initial diagnosis in a single center study. Brain Tumor Res Treat. 2020; 8:77–82. PMID: 33118340.
15. Nadkarni T, Hamilton K, Niazi F, Ward M, Okakpu U, Castellani RJ, et al. Histone-mutant glioma presenting as diffuse leptomeningeal disease. CNS Oncol. 2021; 10:CNS75. PMID: 34469205.
16. Yamasaki K, Yokogami K, Ohta H, Yamashita S, Uehara H, Sato Y, et al. A case of primary diffuse leptomeningeal gliomatosis. Brain Tumor Pathol. 2014; 31:177–181. PMID: 24473978.
17. Dardis C, Milton K, Ashby L, Shapiro W. Leptomeningeal metastases in high-grade adult glioma: development, diagnosis, management, and outcomes in a series of 34 patients. Front Neurol. 2014; 5:220. PMID: 25404928.
18. Intriago B, Danús M, Añaños M, Trampal C, Montero M, Calvo N. 18F-FDG PET detection of spinal leptomeningeal metastases from cerebral glioblastoma multiforme. Eur J Nucl Med Mol Imaging. 2011; 38:1392. PMID: 21394504.
19. Kanai R, Tasaka M, Sejima H, Uchida N, Nakano A, Akiyama Y, et al. Brain stem glioblastoma with multiple large cyst formation and leptomeningeal dissemination in a 4-year-old girl. Brain Dev. 2005; 27:58–61. PMID: 15626543.
20. Pohar S, Taylor W, Chandan VS, Shah H, Sagerman RH. Primary presentation of glioblastoma multiforme with leptomeningeal metastasis in the absence of previous craniotomy: a case report. Am J Clin Oncol. 2004; 27:640–641. PMID: 15577447.
21. Witham TF, Fukui MB, Meltzer CC, Burns R, Kondziolka D, Bozik ME. Survival of patients with high grade glioma treated with intrathecal thiotriethylenephosphoramide for ependymal or leptomeningeal gliomatosis. Cancer. 1999; 86:1347–1353. PMID: 10506724.
22. Reifenberger G, Boström J, Bettag M, Bock WJ, Wechsler W, Kepes JJ. Primary glioblastoma multiforme of the oculomotor nerve. Case report. J Neurosurg. 1996; 84:1062–1066. PMID: 8847574.
23. Shuangshoti S, Shuangshoti S. Primary diffuse leptomeningeal glioblastoma multiforme of brainstem and spinal cord clinically mimicking meningitis: case report and review of literature. J Med Assoc Thai. 1996; 79:403–408. PMID: 8855617.
24. Giordana MT, Bradac GB, Pagni CA, Marino S, Attanasio A. Primary diffuse leptomeningeal gliomatosis with anaplastic features. Acta Neurochir (Wien). 1995; 132:154–159. PMID: 7754854.
25. Norbut AM, Mendelow H. Primary glioblastoma multiforme of the pineal region with leptomeningeal metastases: a case report. Cancer. 1981; 47:592–596. PMID: 6261912.
26. Bae JS, Yang SH, Yoon WS, Kang SG, Hong YK, Jeun SS. The clinical features of spinal leptomeningeal dissemination from malignant gliomas. J Korean Neurosurg Soc. 2011; 49:334–338. PMID: 21887390.
27. Zima LA, Tulpule S, Samson K, Shonka N. Seizure prevalence, contributing factors, and prognostic factors in patients with leptomeningeal disease. J Neurol Sci. 2019; 403:19–23. PMID: 31176194.
28. Alatakis S, Malham GM, Thien C. Spinal leptomeningeal metastasis from cerebral glioblastoma multiforme presenting with radicular pain: case report and literature review. Surg Neurol. 2001; 56:33–37. discussion 37-8. PMID: 11546569.
29. Chan DT, Hsieh SY, Kam MK, Cheung TC, Ng SC, Poon WS. Pattern of recurrence and factors associated with cerebrospinal fluid dissemination of glioblastoma in Chinese patients. Surg Neurol Int. 2016; 7:92. PMID: 27857856.
Fig. 1
Initial contrast enhanced midsagittal MRI reveals prominent leptomeningeal contrast enhancement along the ventral surface of the midbbrain, pons, and medulla oblongata, and dorsal surface of high cervical and medulla (A, white arrows). Coronal view shows right medial temporal lobe round tumor and bilateral basal temporal surface leptomeningeal contrast enhancement (B, white arrows).
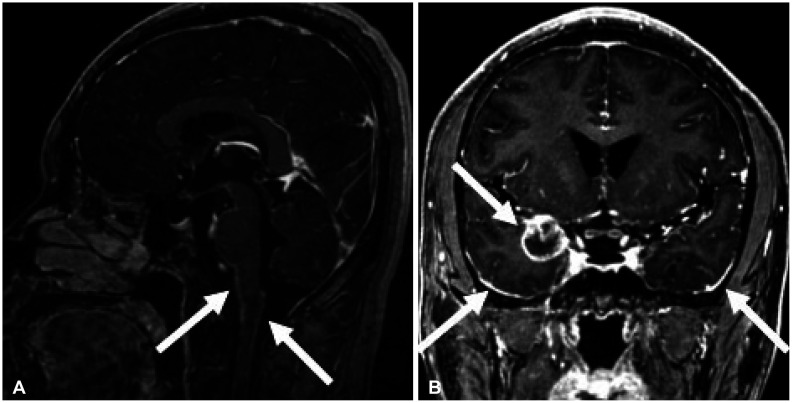
Fig. 2
Whole-body FDG-PET revealing high metabolic uptake lesions on the left cerebral hemisphere (A, white arrows), right anterior temporal lobe tumor (B, white arrow), and spinal cord of T5/6, L1 spinal level (C and D, white arrows).
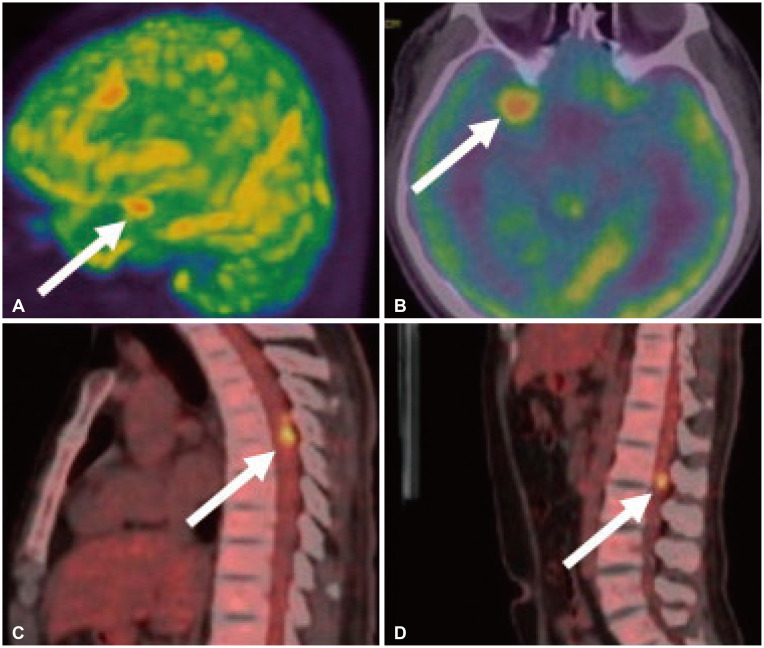
Fig. 3
Right pterional craniotomy and durotomy exposed the anterior Sylvian fissure and veins with normal-looking cortical surface (A, white arrow). At deep Sylvian fissure yellow coating mass with a friable and finely vascularized surface was observed. The lesion was removed near completely through the anterior Sylvian fissure (B, white arrow).
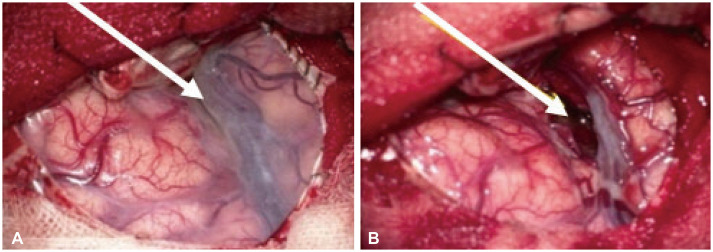
Fig. 4
Histopathologic findings of glioblastoma (Grade 4). The immunohistochemical stain shows positive glial fibrillary acidic protein, and Ki-67 index of more than 30% of tumor cells.(A, ×100; B, ×400). Histological examination of the tumor shows neovascular proliferations and pseudopalisading necrosis (C and D, H&E stain, ×200)
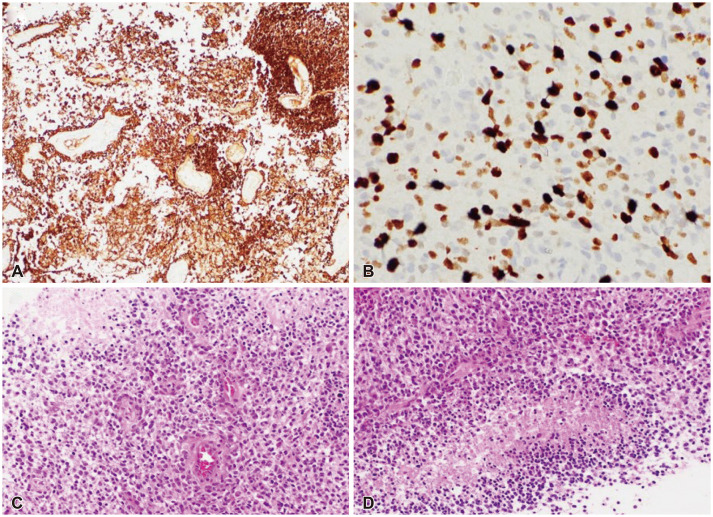
Fig. 5
18F-FDG PET/CT exhibited multifocal high metabolic uptake lesions at the abdomen and pelvic bone (arrows) which were considered as abdominopelvic metastasis (spread) of the glioblastoma via ventriculo-peritoneal shunt.
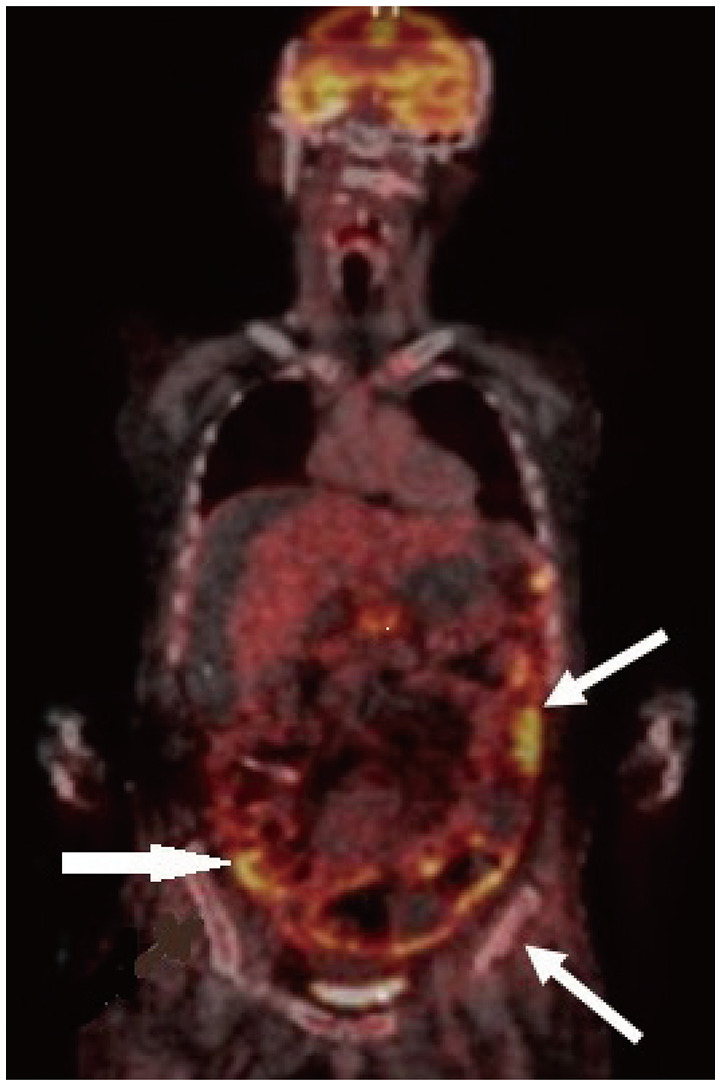
Table 1
Literature summary of primary glioblastoma with LMS
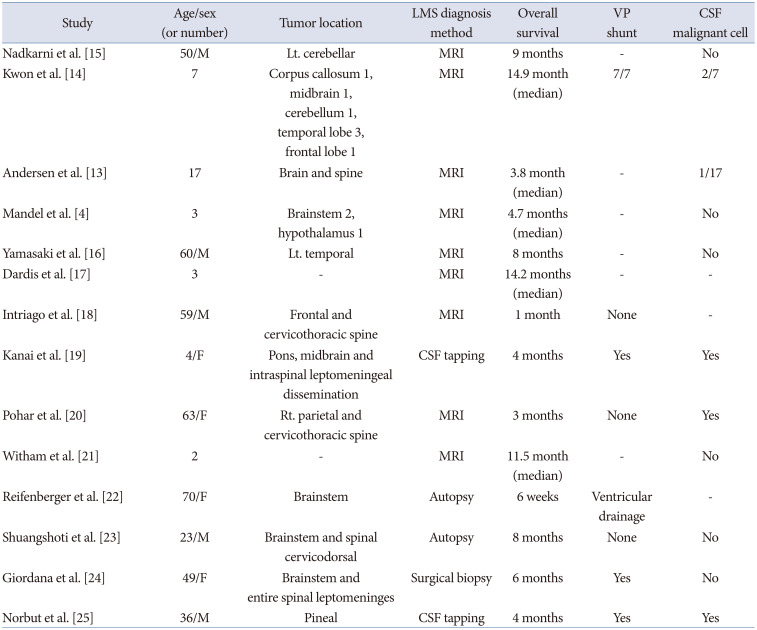
| Study | Age/sex (or number) | Tumor location | LMS diagnosis method | Overall survival | VP shunt | CSF malignant cell |
|---|---|---|---|---|---|---|
| Nadkarni et al. [15] | 50/M | Lt. cerebellar | MRI | 9 months | - | No |
| Kwon et al. [14] | 7 | Corpus callosum 1, midbrain 1, cerebellum 1, temporal lobe 3, frontal lobe 1 | MRI | 14.9 month (median) | 7/7 | 2/7 |
| Andersen et al. [13] | 17 | Brain and spine | MRI | 3.8 month (median) | - | 1/17 |
| Mandel et al. [4] | 3 | Brainstem 2, hypothalamus 1 | MRI | 4.7 months (median) | - | No |
| Yamasaki et al. [16] | 60/M | Lt. temporal | MRI | 8 months | - | No |
| Dardis et al. [17] | 3 | - | MRI | 14.2 months (median) | - | - |
| Intriago et al. [18] | 59/M | Frontal and cervicothoracic spine | MRI | 1 month | None | - |
| Kanai et al. [19] | 4/F | Pons, midbrain and intraspinal leptomeningeal dissemination | CSF tapping | 4 months | Yes | Yes |
| Pohar et al. [20] | 63/F | Rt. parietal and cervicothoracic spine | MRI | 3 months | None | Yes |
| Witham et al. [21] | 2 | - | MRI | 11.5 month (median) | - | No |
| Reifenberger et al. [22] | 70/F | Brainstem | Autopsy | 6 weeks | Ventricular drainage | - |
| Shuangshoti et al. [23] | 23/M | Brainstem and spinal cervicodorsal | Autopsy | 8 months | None | No |
| Giordana et al. [24] | 49/F | Brainstem and entire spinal leptomeninges | Surgical biopsy | 6 months | Yes | No |
| Norbut et al. [25] | 36/M | Pineal | CSF tapping | 4 months | Yes | Yes |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download