INTRODUCTION
Lung cancer is the most common cause of cancer-related deaths in South Korea [
1]. Brain metastasis is a complication and a major concern in the treatment of lung cancer. In fact, in some instances, lung cancer is retrospectively diagnosed in patients presenting with a brain metastatic mass. From a previous report, the incidence of brain metastasis in patients with primary lung cancer was 20% [
2]. Additionally, it has been reported that the incidence of lung cancer with brain metastasis has been increasing in recent years, which places a great burden on public health services [
3]. The treatment options for brain metastasis from primary lung cancer are surgery, conventional radiation therapy, systemic chemotherapy, and stereotactic radiosurgery (SRS). Recently, SRS, including Gamma-Knife (GK) and Cyber-Knife (CK) radiosurgery (RS), has shown good results for the local control of single and multiple brain metastases. However, surgical resection continues to play an important role. Surgery provides immediate relief of mass effect and enables histopathologic examination of the obtained tissue from the procedure. Surgical resection is the essential treatment in large metastasis. However, there are numerous factors to consider in deciding whether patients with brain metastasis from lung cancer should undergo surgical treatment. These factors include current disease state of lung cancer, performance status, tolerability for general anesthesia and craniotomy, expected survival rate after surgery, and postoperative neurological deficits. According to previously published studies, the median survival of patients with lung cancer with brain metastasis was 3–12 months [
4567]. Considering the short survival and the quality of life of patients with cancer, prediction of recurrence and survival is important during surgical decision-making. From a neurosurgeon’s perspective, minimizing local and distant brain recurrence after surgery of metastatic tumor is the critical consideration. Moreover, the need for adjuvant treatment after total resection of the metastatic tumor should also be considered. Herein, we aimed to determine the predictors of recurrence and survival outcomes of patients who were surgically treated for brain metastasis arising from non-small cell lung cancer (NSCLC).
MATERIALS AND METHODS
Patient selection
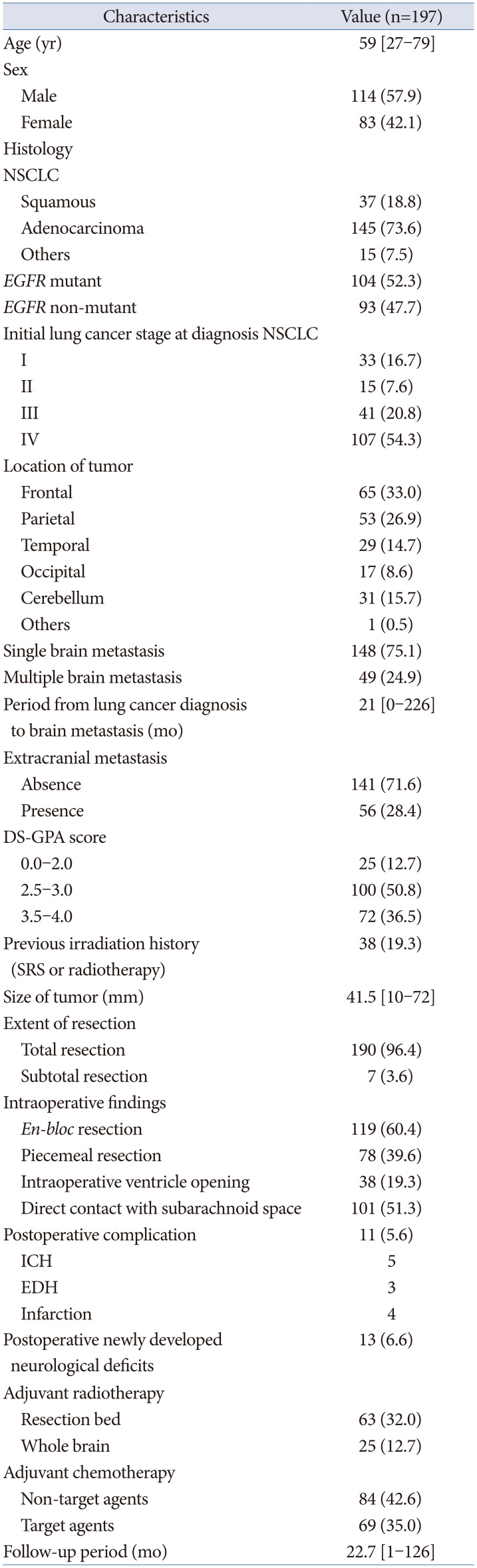
This retrospective study was approved by the Institutional Review Board of Asan Medical Center (approval No.: 2020-0466) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The need for informed consent was waived due to the retrospective nature of the study. Our institutional database was searched for diagnoses of metastatic brain tumors from lung cancer between January 2010 and December 2020. Only patients with brain metastasis from NSCLC who did not undergo surgical resection and those with brain metastasis from NSCLC who underwent microsurgery at our institute were enrolled in the current study. Recurrent tumors with no available information and brain metastasis from small cell lung cancer and other primary tumors were excluded. Altogether, 197 patients were included in this study. The inclusion criteria were as follows: 1) patients with metastatic brain tumors from NSCLC, 2) brain metastasis from NSCLC was treated by surgical resection, and 3) brain metastasis from NSCLC was confirmed via histopathological examination. The tumor size was defined by its maximal diameter in two dimensions.
Treatment and follow-up
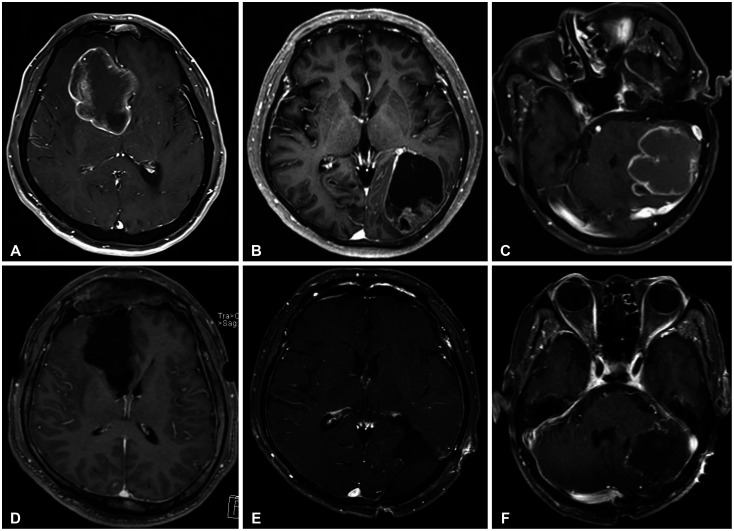
The treatment decision for metastatic brain cancer from NSCLC was established in the integrated lung cancer treatment team. This team consisted of oncologists, radiation-oncologists, and neurosurgeons. The extent of resection in our study was re-defined as follows: gross total resection, complete removal of the tumor as indicated on postoperative MRI, subtotal resection, any incomplete resection of the tumor with less than 10% of the tumor visible as a remnant on postoperative MRI. The surgical methodology is classified as “en-bloc resection” and “piecemeal resection.” The definition of “en-bloc resection” is removal of the entirely of a tumor without violating tumor capsule and the definition of “piecemeal resection” is removal of the tumor divided into small pieces.
SRS, including GKRS and CKRS, were additionally performed for residual tumors. GKRS was performed using a Leksell GK perfexion (Elekta, Stockholm, Sweden) from December 2011 until the present. Dose planning for the GKRS treatment involved MRI analysis in the Leksell gamma plan. The marginal prescription doses ranged from 20 Gy to 24 Gy. The Robotic Radiosurgery System Version 9.0 (Accuray, Sunnyvale, CA, USA) was used for CK treatments. For CK planning, CT images with a very thin slice (0.625-mm thickness with no slice gap) were obtained and fused with gadolinium-enhanced axial and coronal three-dimensional T1-magnetization-prepared rapid acquisition gradient-echo MR images (1.0-mm slice) in the Accuray MultiPlan system (version 4.5; Accuray). The median prescription dose was 35 Gy (range, 25–41 Gy). The dose was administered in three or five daily fractions. Conventional radiation therapy was administered for the surgical cavity or whole brain, the prescription dose was 30 Gy, which was fractionated by 2 Gy or 3 Gy.
Initial follow-ups involved clinical evaluation and an MRI in the immediate postoperative period (within 48 h), at the first and third months, and every 3 months thereafter.
Statistical analyses
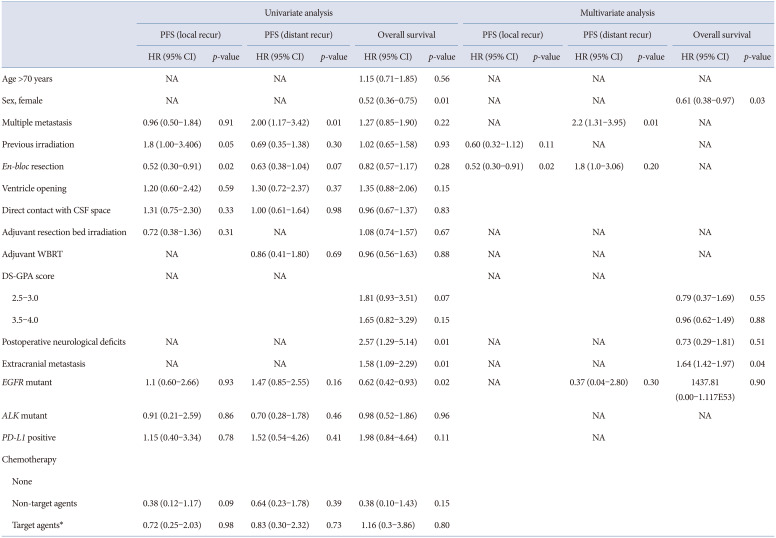
The overall survival (OS), progression-free survival (PFS), and prognostic factors were evaluated. The OS and PFS outcomes were analyzed using the Kaplan-Meier survival analysis, and subgroup comparisons were performed using the log-rank tests. Potential prognostic factors for PFS, including age, sex, multiple metastases, adjuvant resection bed irradiation, adjuvant whole-brain radiation therapy (WBRT), period from lung cancer diagnosis to the occurrence of brain metastasis, postoperative neurological deficits, and immunohistochemical findings in NSCLC, were analyzed using the Cox-proportional hazards model. The proportional hazards assumption was confirmed via testing of the Schoenfeld residuals, and no relevant violations were found. All statistical analyses were conducted using the SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). A p-value <0.05 was considered statistically significant.
DISCUSSION
Lung cancer is the most frequent source of metastatic brain tumors. It occurs in 17%–65% of patients with primary lung cancer [
38]. The rate of lung cancer brain metastasis has been increasing in recent years [
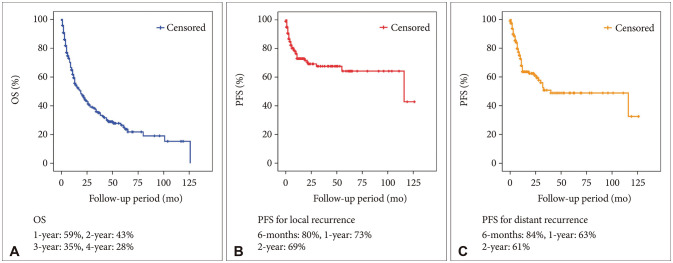
3]. According to previously published studies, the prognosis for patients with metastatic brain cancer from lung cancer is poor, with the median OS reported to be 1 to 12 months despite treatment [
456]. However, there are significant advances in the management of lung cancer. In our study, the median OS was over 12 months.
To prolong the life and improve the quality of life of patients with metastatic brain cancer from lung cancer, there should be relief of neurological symptoms and intracranial hypertension. The treatment of brain metastasis is critical in patients with lung cancer. Thus, the role of neurosurgeons is considerably important in the treatment of brain metastasis from lung cancer. From the perspective of a neurosurgeon in brain metastasis treatment, decreasing local and distant recurrence while maintaining or improving the quality of life of patients are the most important considerations. Surgery, SRS, conventional radiation therapy, and systemic chemotherapy are the possible treatment strategies in brain metastasis. The efficacy of radiosurgery for small-sized multiple brain metastasis has been proven [
7]. Moreover, Chon et al. [
9] reported the feasibility of fractionated radiosurgery for medium-sized brain metastasis. For medium to large tumors, surgical resection is still an essential tool. However, surgery is the only option for large tumors with mass effects, which should be confirmed pathologically. In these cases, progression has been observed even after radiosurgery or radiotherapy. In our center, decision of surgical treatment of BM has been established after multidisciplinary discussion between the neurosurgeons, radiation-oncologists and medical oncologists. Generally, SRS including GKRS or CKRS, are considered primarily for small tumor. In medium sized tumor, SRS is primarily considered for tumor which do not show mass effect, deep or eloquent location tumor. However, surgical resection is primarily considered for large tumor or rapid alleviating neurological symptom or tissue histopathological examination. Recently, we performed surgical resection in patients with single metastasis and multiple metastases. Surgery with adjunctive radiosurgery or radiotherapy were performed to prolong the life and to relieve symptoms related to brain metastasis.
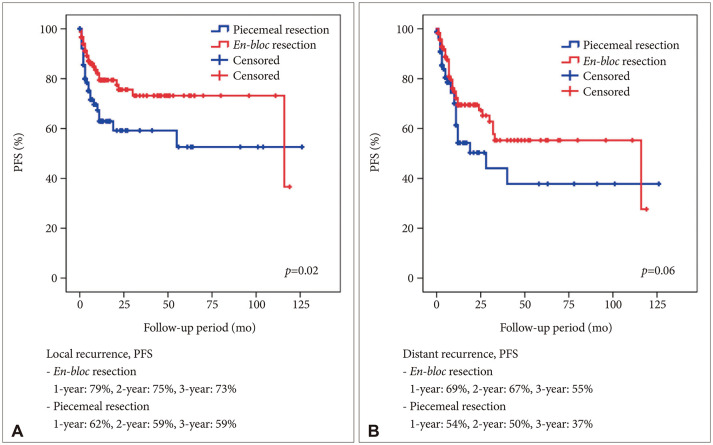
In this study, we focused on analyzing possible predictors for local and distant recurrence of brain metastatic tumors from NSCLC from a neurosurgeon’s perspective. Total resection of metastatic brain tumor was performed in 97% of the patients. To reduce local recurrence,
en-bloc resection was performed.
En-bloc resection resulted in better outcomes compared with those of piecemeal resection [
10]. In our series, we did gross total resection without plus normal tissue resection. However, concordant with a previous report,
en-bloc resection resulted in a better local control of NSCLC brain metastasis in our study. Furthermore, Yoo et al. [
11] reported that a plus 5 mm adjacent white matter resection reduced local recurrence.
En-bloc tumor resection usually involves tumor resection in the gliotic plane or normal white matter adjacent the tumor. Furthermore, we found that supra-marginal resection leads to less local recurrence after tumor resection. Although tumor resection was not feasible in all tumors, “
en-bloc”-fashion resection plus white matter was pursued for non-eloquent area tumors to reduce the local recurrence rate. There has not been concrete evidence of impact of tumor cystic fluid for tumor recurrence or dissemination. However, in cystic metastatic tumor surgery, we performed cystic fluid aspiration before tumor resection to prevent rupture of tumor cyst. After cyst aspiration, it is not too difficult to perform extra-tumoral dissection and “
en-bloc” resection. Theoretically, ventricular opening or direct contact between the tumor and the subarachnoid space, including cortically protruding tumor, protruding tumor into the cisternal space, and tumor adherent to the dura, may act as routes where the tumor may spread. In primary malignant brain tumors, ventricular opening during brain tumor resection increased the incidence of distant recurrence and leptomeningeal seeding [
12]. However, in this study, ventricular opening during surgery and direct contact between the tumor and the subarachnoid space were not associated with distant recurrence and leptomeningeal seeding.
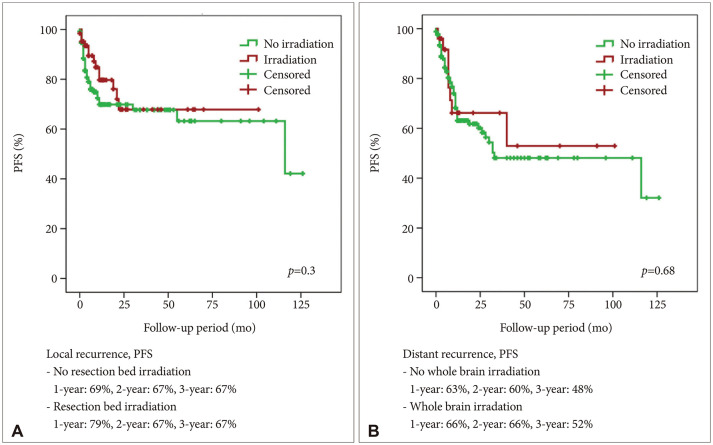
Postoperative irradiation, including SRS and conventional radiation therapy, could be applied in the resection cavity to reduce local recurrence. SRS and WBRT showed comparable results in terms of local recurrence in brain metastasis [
613]. In a previous report, the efficacies of SRS and WBRT were comparable [
61314]. WBRT has been needed for disseminated metastasis and it has performed a unique role. However, considering the neuro-cognitive complications such as learning and memory dysfunction, SRS may be a more suitable tool for resection bed irradiation [
15]. Theoretically, resection bed irradiation may provide good local control but poor distant recurrence control. However, in our series, resection bed radiotherapy and radiosurgery did not affect the local recurrence. In addition, postoperative WBRT did not affect the incidence of distant recurrence.
The results of our study should be cautiously interpreted. In this study, it was not implicated that adjuvant radiotherapy and radiosurgery might not have been mandatory after surgical resection for patients with metastatic brain cancer from NSCLC since our study did not follow a single treatment consensus and since the patients did not receive the same chemotherapeutic regimen. In the previous studies, only WBRT for brain metastasis influenced survival from 1 to 3 months [
1617]. Applying WBRT for reducing distant recurrence in lung cancer brain metastasis, considering its survival benefit and neurocognitive impairment, was still debatable. Although it should be cautiously interpreted, according to our result, WBRT did not improve the PFS for distant recurrence.
At our institute, there was no consensus for applying adjuvant radiotherapy or SRS. Instead, it depended on the opinion of individual oncologists. Standard cytotoxic chemotherapy used in lung cancer, mainly platinum agents, was not effective metastatic brain cancer from NSCLC due to poor penetration of the blood-brain barrier [
4]. On the other hand, molecular-targeted therapy showed promising effects on patients with metastatic brain cancer from NSCLC [
418].
EGFR mutation,
ALK rearrangement, and
PD-L1 are targetable mutations. They have shown effectiveness in intracranial disease control. Moreover, a recent clinical trial reported promising results of
EGFR-targeted therapy for leptomeningeal diseases [
12]. However,
EGFR-targeted therapy is not suitable for symptomatic and immediate life-threatening brain metastasis. Nevertheless, it could still be applied for small metastasis and disseminated disease. At our institute, we used a molecular-targeted agent after surgical resection of metastatic brain tumor according to the status of targetable gene mutation. We evaluated the difference of local recurrence and distant recurrence concerning
EGFR,
ALK-1, and
PD-L1 in the NSCLC group. There was no statistically significant difference in local and distant recurrence. Recently, molecular target agents have been shown the efficacy for control of brain metastatic tumors. However, there has been lack of studies for impact of molecular target agents for local or distant recurrence after surgery for brain metastasis [
19]. The efficacy of molecular target agent after brain metastatic tumor resection should be evaluated in future studies.
In this study, only
en-bloc resection affected the prognosis for local recurrence, while only multiple brain metastasis affected the prognosis for distant recurrence. In cases of multiple brain metastasis, short-term follow-up brain imaging was needed to detect another metastasis. In terms of OS, female sex and extracranial metastasis were statistically significant prognostic factors. Female sex was already known as a favorable prognostic factor of lung cancer [
20].
The maintenance of the quality of life of patients with cancer is an important consideration. Considering the lower chance of cure of disease in patients with brain metastasis from lung cancer, the postoperative complication significantly impacting the performance status of patients should be minimized. In recent studies, the eloquent location of metastatic brain tumors could be managed using radiosurgery [
79]. A declining performance status due to postoperative neurological deficits not only restricted further chemotherapy or radiation therapy but also led to other medical complications, which jeopardized the survival of patients with lung cancer. In our study, the postoperative complication group showed a significantly lower OS rate. We should minimize the postoperative complication in brain metastasis surgery and consider that the complication critically influences survival.
Our current study had some limitations. First, it was a retrospective investigation that included 197 patients with lung cancer brain metastasis. Along with the limitations inherent to any retrospective design, this precluded less meaningful multivariate analysis of survival outcomes or predictors of local and distant recurrence. Moreover, this study series spanned around 10 years; therefore, the treatment methods varied. The management strategy of lung cancer brain metastasis varied according to individual neurosurgeons, which made it challenging to conclude on the optimal intervention strategies and outcomes in these cases. However, we believe that the data from our current single-center series make a valuable contribution to the available literature on these extremely rare tumors and to a future meta-analysis of this disease.
In summary, surgery of brain metastasis from NSCLC is an important treatment method to improve survival and defer life-threatening events during lung cancer treatment. En-bloc resection may reduce local recurrence after surgical resection of brain metastasis. Ventricular opening and contact between the tumor and subarachnoid space did not show a statistically significant result for distant recurrence and leptomeningeal seeding. Resection bed irradiation and WBRT did not show effectiveness to reduce local and distant recurrence. However, it should be cautiously interpreted. Multiple metastasis was only meaningful factor for distant recurrence. Future large-sized study should be conducted.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download