Abstract
Over the last two decades, numerous studies have investigated the presence of human cytomegalovirus (CMV) within glioblastoma or gliomas; however, the results are severely conflicting. While a few researchers have suggested the potential benefits of cytotoxic T lymphocyte or dendritic cell-based vaccines for recurrent or newly diagnosed glioblastoma patients, several studies did not at all agree with the existence of CMV in glioblastoma cells. In this review, we summarized the conflicting results and issues about the detection of CMV in glioblastoma or glioma patients. We also provided the clinical data of published and unpublished clinical trials using CMV-specific immunotherapy for glioblastomas.
Viral antigens expressed within cancer cells have long been investigated as attractive immunological targets in regard to tumor-specific cancer immunotherapy, including cytotoxic T lymphocyte (CTL) or dendritic cell (DC)-based vaccines, because a viral antigen, as a non-self antigen, can elicit potent antitumor immunity in vivo and ex vivo, compared to tumor-associated antigens. Over the last two decades, numerous studies have investigated the presence of cytomegalovirus (CMV) within glioblastoma or gliomas; however, the results are severely conflicting. While a few researchers have suggested the potential benefits of CTL or DC vaccines for recurrent or newly diagnosed glioblastoma patients, several studies did not at all agree with the existence of CMV in glioblastoma cells [123456789101112]. In this context, we summarized the conflicting results and issues about the detection of CMV in glioblastoma or glioma patients. We also provided the clinical data of published and unpublished clinical trials using CMV-specific immunotherapy for glioblastomas.
Electronic databases, PubMed, Google Scholar, Directory of Open Access Journals, and Web of Science were searched from January 2022 to February 2022. Database searches included the following key words: ‘glioblastoma or glioma’ and ‘cytomegalovirus or CMV.’ Two researchers (CS and JA) extracted the relevant information and validated their inclusion in the current review.
The presence of human CMV in patients with malignant gliomas was first reported using the methods of immunohistochemistry (IHC) and in situ hybridization (ISH) by Cobbs et al. [13]. Since then, various methods have been utilized to detect human CMV from glioblastoma specimens. Mitchell et al. [14] was the first to detect CMV DNA using polymerase chain reaction (PCR), analyzing glioblastoma specimens and peripheral blood. Western blot, flow cytometry, and next-generation sequencing (NGS) also utilized. Among these methods, IHC is most frequently used to detect human CMV in glioblastoma samples. Based on several studies suggesting the relationship between human CMV and glioblastoma, consensus on the role of human CMV in glioblastoma was made in 2011 [15]. For high sensitivity in detection, a precise method involving cell culture, immunostaining, and RNA/protein extraction from glioblastoma tissue was also proposed by Cobbs et al. [16]. Nevertheless, results of recent studies are conflicting. Detailed results according to the detection method are described in Tables 1, 2, 3, 4.
A total of 36 studies have evaluated the presence of proteins in paraffin sections of glioblastomas and/or gliomas using IHC methods and 23 studies suggested the presence of CMV proteins in patient’s specimens, while 13 studies did not demonstrate the presence of CMV proteins (Table 1) [127101314171819202122232425262728293031323334353637383940414243444546]. When using IHC, the median detection rates of CMV protein for gliomas or glioblastoma was 77.5% (range, 2.1% to 100%), and median detection rates for glioblastoma only was 90.5% (range, 2.1% to 100%). The detection rates of CMV proteins seemed higher in glioblastoma than in gliomas [13171819202930313233343536]. Immediate-early proteins (IEs) and phosphoprotein 65 (pp65) are popular targets when using IHC. Among the 36 total studies, 28 studies targeted IEs (17 studies targeted IE-1 specifically) and 16 studies targeted pp65.
In addition, 14 studies utilized ISH to detect CMV DNA or mRNA in paraffin sections of glioblastoma and/or gliomas [113141718232731323841424344]. Seven studies showed positive results, while seven studies did not find CMV genomic products (Table 2). When using ISH, the median detection rate of CMV genomic products for gliomas or glioblastoma was 64.9% (range, 5.3% to 100%) [13171823313238].
Thirty-one studies utilized PCR to detect CMV genes within tumors, and 16 studies showed positive results (range, 16.4% to 100%), while 15 studies failed to find CMV genome markers (Table 3) [12810121419252627283133363739404142434446474849505152535455]. Utilizing primers specific for the CMV glycoprotein B (gB) gene was the first attempt to detect CMV by PCR [1]. Primers of gB, IE, and pp65 were utilized in 15, 8, and 4 studies each, respectively.
Researchers also utilized NGS to detect CMV genes within tumors based on their own samples, as well as public database. The most common data source is The Cancer Genome Atlas (TCGA) and National Center for Biotechnology Information (NCBI). Out of eight studies, four studies downloaded NGS datasets from the TCGA Cancer Genomics Hub repository (CGHub, now migrated to Genomic Data Commons; https://portal.gdc.cancer.gov) and three studies from NCBI Sequence Read Archive (SRA; https://www.ncbi.nlm.nih.gov/sra) or Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) (Table 4) [34569113756]. Unlike other methods, all studies using NGS failed to demonstrate the presence of CMV genes. There was only one positive case from a study, but it may be explained by the contamination of CMV protomer gene [37]. In summary, studies using, IHC, ISH, or PCR favored the presence of CMV in glioblastoma or gliomas, while studies using NGS method did not find the presence of CMV.
The difference between studies may be explained by several reasons. First, CMV proteins and nucleotides could be readily detected if the entire protocol is optimized as suggested [1516]. In IHC, paraffin blocks of fresh brain autopsy specimens must be sectioned in 6 µm slices. Application of pepsin or trypsin at 37℃ for 4–6 min and of citrate at 85℃–90℃ for 2–4 min followed by washing in a 45℃–50℃ water bath for 2.5 hour should be performed carefully to avoid damage to viral antigens. Han et al. [33], following the methodology of Cobbs et al. [16], showed a high detection rate using IHC (82.1% and 68.4% in glioblastoma for IE-1 and pp65, respectively). In contrast, some studies utilizing thin formalin-fixed paraffin-embedded tissue sections (3–4 µm) or an automated immunostainer demonstrated low detection rates [364344]. Yang et al. [42] failed to detect IE protein in 116 samples using the methodology of Cobbs et al. [16]. The low detection limit of IHC, small sample size, and measurement error may explain the false negative results as well [26]. Second, the blood positivity of the CMV may also contribute to the detection results for human CMV. All eight studies showing the seropositive in patient’s blood, found the presence of CMV in tumors [1419233039464951].
Nevertheless, the results between studies are conflicting and not consistent according to detection methods, even within a single study. Further and larger studies to clarify this controversy are needed.
Over the past several decades, several viruses have turned out to elicit oncogenesis. Including human papillomavirus that causes cervical cancer and hepatitis C virus that causes liver cancer, oncogenic viruses are responsible for 10% to 15% of human cancers [57]. These viruses directly affect healthy cells and cause cancer transformation through spreading its nucleic acids. Meanwhile, other viruses, including human CMV, are known to cause cancer in a more indirect manner, which is known as onco-modulatory effect. In other words, human CMV infection, unlike oncovirus, is known to enhance malignancy via formation of tumor-related microenvironment [58].
Human CMV has recently been suggested to have a onco-modulatory role in several brain malignancies including glioma, medulloblastoma, and neuroblastoma [5960]. Onco-modulatory effects are defined as contributing to increase the extent of malignancy. In detail, human CMV infection induced the cells to be more vulnerable to carcinogenic materials due to lack of adhesion molecules in neurons, which are more aggravated when CMV re-activation occurred more frequently [5860]. In addition, human CMV-infected glioma cells showed stem-like characteristics with increased IE protein expression [34394561]. Several studies suggest specific onco-modulatory roles of CMV-infected glioma. These roles include self-sufficiency in growth signals, insensitivity to anti-growth signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, tissue invasion and metastasis, deregulation of cellular energetics, avoiding immune destruction, tumor-promoting inflammation, and genome instability and mutation [5859].
Until now, CMV-specific immunotherapy mainly includes autologous CMV-specific CTL or DC-based therapeutic vaccines. The first study using autologous CMV-specific CTL for recurrent glioblastoma was published in 2014 [62]. Autologous CMV-specific CTL generated by synthetic peptide epitope stimulation ex vivo was administered for 13 recurrent glioblastoma patients without severe adverse toxicities. The clinical outcomes suggested a substantially increased median survival (overall survival more than 57 weeks), compared to that of historical glioblastoma patients. Another study published in 2020 using autologous CTL for newly diagnosed glioblastoma showed the clinical experiences of 25 patients treated with CTL [63]. The group that received CTL before progression demonstrated longer overall survival (23 versus 14 months) than those who received CTL after progression.
Three studies reported the clinical outcomes of autologous CMV-specific DC-based vaccines for newly diagnosed glioblastoma patients. The first study, using a CMV-DC vaccine published in 2015, included 12 patients with newly diagnosed glioblastoma. The study showed better overall survival and progression-free survival (with hazard ratios of 0.620 in overall survival) [64]. Also, in the group treated with pp65-pulsed DC vaccine with tetanus and diphtheria toxoid (Td), increasing migration of DC toward lymph node was found than in a group treated with an unpulsed DC vaccine. A second study published in 2018 included 15 patients with newly diagnosed glioblastoma who received an autologous CMV-specific CTL and pp65 pulsed CMV-DC vaccine [65]. Patients treated with CTL therapy followed by CMV-DC vaccine showed better overall survival and progression free survival (13.4 month overall survival and 8.1 month progression-free survival after recurrence). These two studies suggested that the pp65-specific DC vaccine was associated with increased CMV-specific T cell frequency as well as survival outcomes, when combined with Td toxin or autologous CTL. Another study using a CMV-specific DC vaccine combined with the standard of care including concomitant chemoradiation followed by dose-intensified temozolomide, enrolled 14 patients with newly diagnosed glioblastoma [66]. When the patients were treated with pp65-specific DC vaccine with granulocyte macrophage-colony stimulating factor (GM-CSF) after temozolomide, the median overall survival was 41.1 months and progression-free survival 25.3 months. Table 5 summarizes the published results of clinical trials of CMV-specific immunotherapy for glioblastoma [6263646566].
Unpublished clinical trials of CMV-specific immunotherapy include various combinational strategies. In detail, among four clinical trials using CMV-specific CTL therapy (NCT00990496, NCT01205334, NCT01109095, NCT02661282), one recent trial applied CMV-specific CTL with an engineered HER-2 chimeric antigen receptor (NCT01109095). Clinical trials using CMV-specific DC-based vaccines with various adjuvants such as GM-CSF (NCT04963413), Td toxin (NCT03615404, NCT02465268, NCT03927222), or cancer drugs including IL-2 receptor antagonist (NCT00626483, NCT02366728), anti-CD27 antibody (NCT03688178), and anti-PD-1 inhibitor (NCT02529072) are currently being investigated. Another type of CMV-specific vaccine including enveloped virus-like particle vaccine (VBI-1901), or pp65-specific monocyte vaccine are also being investigated (NCT03382977, NCT04741984). The summary of unpublished clinical trials of CMV-specific immunotherapy for glioblastoma is contained in Table 6.
As one of the novel immunotherapeutic strategies, clinical approaches using CMV-specific CTL and/or DC-vaccines have been tested. However, there are some limitations of previous studies. First, numerous studies have tried to evaluate the presence of CMV within glioblastoma; however, consolidative results for the presence of CMV within glioblastoma are needed. Second, the onco-modulatory role of CMV for gliomagenesis is unknown. Third, clinical trials have suggested positive clinical outcomes, but additional larger and randomized studies are needed. As viral antigens can elicit one of the most powerful immune repones, the presence of viral antigens within tumor cells can be an attractive immuno-therapeutic target for various cancer types. Therefore, further translational studies are needed to support the presence of CMV and the onco-modulatory role in gliomagenesis.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article.
References
1. Lau SK, Chen YY, Chen WG, Diamond DJ, Mamelak AN, Zaia JA, et al. Lack of association of cytomegalovirus with human brain tumors. Mod Pathol. 2005; 18:838–843. PMID: 15578071.
2. Poltermann S, Schlehofer B, Steindorf K, Schnitzler P, Geletneky K, Schlehofer JR. Lack of association of herpesviruses with brain tumors. J Neurovirol. 2006; 12:90–99. PMID: 16798670.
3. Khoury JD, Tannir NM, Williams MD, Chen Y, Yao H, Zhang J, et al. Landscape of DNA virus associations across human malignant cancers: analysis of 3,775 cases using RNA-Seq. J Virol. 2013; 87:8916–8926. PMID: 23740984.
4. Tang KW, Alaei-Mahabadi B, Samuelsson T, Lindh M, Larsson E. The landscape of viral expression and host gene fusion and adaptation in human cancer. Nat Commun. 2013; 4:2513. PMID: 24085110.
5. Cimino PJ, Zhao G, Wang D, Sehn JK, Lewis JS Jr, Duncavage EJ. Detection of viral pathogens in high grade gliomas from unmapped next-generation sequencing data. Exp Mol Pathol. 2014; 96:310–315. PMID: 24704430.
6. Cosset É, Petty TJ, Dutoit V, Cordey S, Padioleau I, Otten-Hernandez P, et al. Comprehensive metagenomic analysis of glioblastoma reveals absence of known virus despite antiviral-like type I interferon gene response. Int J Cancer. 2014; 135:1381–1389. PMID: 24347514.
7. Solomon IH, Ramkissoon SH, Milner DA Jr, Folkerth RD. Cytomegalovirus and glioblastoma: a review of evidence for their association and indications for testing and treatment. J Neuropathol Exp Neurol. 2014; 73:994–998. PMID: 25289896.
8. Lin CT, Leibovitch EC, Almira-Suarez MI, Jacobson S. Human herpesvirus multiplex ddPCR detection in brain tissue from low- and high-grade astrocytoma cases and controls. Infect Agent Cancer. 2016; 11:32. PMID: 27462365.
9. Strong MJ, Blanchard E 4th, Lin Z, Morris CA, Baddoo M, Taylor CM, et al. A comprehensive next generation sequencing-based virome assessment in brain tissue suggests no major virus - tumor association. Acta Neuropathol Commun. 2016; 4:71. PMID: 27402152.
10. Taha MS, Abdalhamid BA, El-Badawy SA, Sorour YM, Almsned FM, Al-Abbadi MA. Expression of cytomegalovirus in glioblastoma multiforme: myth or reality? Br J Neurosurg. 2016; 30:307–312. PMID: 26742571.
11. Johnson TS, Abrams ZB, Mo X, Zhang Y, Huang K. Lack of human cytomegalovirus expression in single cells from glioblastoma tumors and cell lines. J Neurovirol. 2017; 23:671–678. PMID: 28695489.
12. Strojnik T, Duh D, Lah TT. Prevalence of neurotropic viruses in malignant glioma and their onco-modulatory potential. In Vivo. 2017; 31:221–229. PMID: 28358704.
13. Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002; 62:3347–3350. PMID: 12067971.
14. Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008; 10:10–18. PMID: 17951512.
15. Dziurzynski K, Chang SM, Heimberger AB, Kalejta RF, McGregor Dallas SR, Smit M, et al. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol. 2012; 14:246–255. PMID: 22319219.
16. Cobbs CS, Matlaf L, Harkins LE. Methods for the detection of cytomegalovirus in glioblastoma cells and tissues. Methods Mol Biol. 2014; 1119:165–196. PMID: 24639224.
17. Sabatier J, Uro-Coste E, Pommepuy I, Labrousse F, Allart S, Trémoulet M, et al. Detection of human cytomegalovirus genome and gene products in central nervous system tumours. Br J Cancer. 2005; 92:747–750. PMID: 15700045.
18. Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008; 116:79–86. PMID: 18351367.
19. Ding D, Han S, Wang Z, Guo Z, Wu A. Does the existence of HCMV components predict poor prognosis in glioma? J Neurooncol. 2014; 116:515–522. PMID: 24395349.
20. Libard S, Popova SN, Amini RM, Kärjä V, Pietiläinen T, Hämäläinen KM, et al. Human cytomegalovirus tegument protein pp65 is detected in all intra- and extra-axial brain tumours independent of the tumour type or grade. PLoS One. 2014; 9:e108861. PMID: 25268364.
21. Slinger E, Maussang D, Schreiber A, Siderius M, Rahbar A, Fraile-Ramos A, et al. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Sci Signa. 2010; 3:ra58.
22. Lucas KG, Bao L, Bruggeman R, Dunham K, Specht C. The detection of CMV pp65 and IE1 in glioblastoma multiforme. J Neurooncol. 2011; 103:231–238. PMID: 20820869.
23. Ghazi A, Ashoori A, Hanley PJ, Brawley VS, Shaffer DR, Kew Y, et al. Generation of polyclonal CMV-specific T cells for the adoptive immunotherapy of glioblastoma. J Immunother. 2012; 35:159–168. PMID: 22306904.
24. Rahbar A, Stragliotto G, Orrego A, Peredo I, Taher C, Willems J, et al. Low levels of human cytomegalovirus infection in glioblastoma multiforme associates with patient survival; -a case-control study. Herpesviridae. 2012; 3:3. PMID: 22424569.
25. Rahbar A, Orrego A, Peredo I, Dzabic M, Wolmer-Solberg N, Strååt K, et al. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J Clin Virol. 2013; 57:36–42. PMID: 23391370.
26. Baumgarten P, Michaelis M, Rothweiler F, Starzetz T, Rabenau HF, Berger A, et al. Human cytomegalovirus infection in tumor cells of the nervous system is not detectable with standardized pathologico-virological diagnostics. Neuro Oncol. 2014; 16:1469–1477. PMID: 25155358.
27. Yamashita Y, Ito Y, Isomura H, Takemura N, Okamoto A, Motomura K, et al. Lack of presence of the human cytomegalovirus in human glioblastoma. Mod Pathol. 2014; 27:922–929. PMID: 24336154.
28. Bianchi E, Roncarati P, Hougrand O, Guérin-El Khourouj V, Boreux R, Kroonen J, et al. Human cytomegalovirus and primary intracranial tumours: frequency of tumour infection and lack of correlation with systemic immune anti-viral responses. Neuropathol Appl Neurobiol. 2015; 41:e29–e40. PMID: 25041908.
29. Huang R, Qian D, Hu M, Zhang X, Song J, Li L, et al. Association between human cytomegalovirus infection and histone acetylation level in various histological types of glioma. Oncol Lett. 2015; 10:2812–2820. PMID: 26722247.
30. Shamran HA, Kadhim HS, Hussain AR, Kareem A, Taub DD, Price RL, et al. Detection of human cytomegalovirus in different histopathological types of glioma in Iraqi patients. Biomed Res Int. 2015; 2015:642652. PMID: 25710012.
31. Stangherlin LM, Castro FL, Medeiros RS, Guerra JM, Kimura LM, Shirata NK, et al. Human cytomegalovirus DNA quantification and gene expression in gliomas of different grades. PLoS One. 2016; 11:e0159604. PMID: 27458810.
32. Xing Y, Wang Y, Wang S, Wang X, Fan D, Zhou D, et al. Human cytomegalovirus infection contributes to glioma disease progression via upregulating endocan expression. Transl Res. 2016; 177:113–126. PMID: 27474433.
33. Han S, Deng J, Wang Z, Liu H, Cheng W, Wu A. Decreased human leukocyte antigen A*02:01 frequency is associated with risk of glioma and existence of human cytomegalovirus: a case-control study in Northern China. Cancer Immunol Immunother. 2017; 66:1265–1273. PMID: 28523518.
34. Hu M, Wang B, Qian D, Wang M, Huang R, Wei L, et al. Human cytomegalovirus immediate-early protein promotes survival of glioma cells through interacting and acetylating ATF5. Oncotarget. 2017; 8:32157–32170. PMID: 28473657.
35. Qin Z, Zhang L, Xu Y, Zhang X, Fang X, Qian D, et al. TLR3 regulates PD-L1 expression in human cytomegalovirus infected glioblastoma. Int J Clin Exp Pathol. 2018; 11:5318–5326. PMID: 31949612.
36. Maleki F, Sadigh ZA, Sadeghi F, Muhammadnejad A, Farahmand M, Parvin M, et al. Human cytomegalovirus infection in Iranian glioma patients correlates with aging and tumor aggressiveness. J Med Virol. 2020; 92:1266–1276. PMID: 31944314.
37. Tang KW, Hellstrand K, Larsson E. Absence of cytomegalovirus in high-coverage DNA sequencing of human glioblastoma multiforme. Int J Cancer. 2015; 136:977–981. PMID: 24961996.
38. Wakefield A, Pignata A, Ghazi A, Ashoori A, Hegde M, Landi D, et al. Is CMV a target in pediatric glioblastoma? Expression of CMV proteins, pp65 and IE1-72 and CMV nucleic acids in a cohort of pediatric glioblastoma patients. J Neurooncol. 2015; 125:307–315. PMID: 26341370.
39. Bahador M, Gras Navarro A, Rahman MA, Dominguez-Valentin M, Sarowar S, Ulvestad E, et al. Increased infiltration and tolerised antigen-specific CD8+ TEM cells in tumor but not peripheral blood have no impact on survival of HCMV+ glioblastoma patients. Oncoimmunology. 2017; 6:e1336272. PMID: 28919997.
40. Garcia-Martinez A, Alenda C, Irles E, Ochoa E, Quintanar T, Rodriguez-Lescure A, et al. Lack of cytomegalovirus detection in human glioma. Virol J. 2017; 14:216. PMID: 29116009.
41. Holdhoff M, Guner G, Rodriguez FJ, Hicks JL, Zheng Q, Forman MS, et al. Absence of cytomegalovirus in glioblastoma and other high-grade gliomas by real-time PCR, immunohistochemistry, and in situ hybridization. Clin Cancer Res. 2017; 23:3150–3157. PMID: 28034905.
42. Yang CF, Ho HL, Lin SC, Hsu CY, Ho DM. Detection of human cytomegalovirus in glioblastoma among Taiwanese subjects. PLoS One. 2017; 12:e0179366. PMID: 28594901.
43. Loit MP, Adle-Biassette H, Bouazza S, Mazeron MC, Manivet P, Lehmann-Che J, et al. Multimodal techniques failed to detect cytomegalovirus in human glioblastoma samples. J Neurovirol. 2019; 25:50–56. PMID: 30397828.
44. Limam S, Missaoui N, Hmissa S, Yacoubi MT, Krifa H, Mokni M, et al. Investigation of human cytomegalovirus and human papillomavirus in glioma. Cancer Invest. 2020; 38:394–405. PMID: 32643440.
45. Wen L, Zhao F, Qiu Y, Cheng S, Sun JY, Fang W, et al. Human cytomegalovirus DNA and immediate early protein 1/2 are highly associated with glioma and prognosis. Protein Cell. 2020; 11:525–533. PMID: 32189197.
46. Zavala-Vega S, Castro-Escarpulli G, Hernández-Santos H, Salinas-Lara C, Palma I, Mejía-Aranguré JM, et al. An overview of the infection of CMV, HSV 1/2 and EBV in Mexican patients with glioblastoma multiforme. Pathol Res Pract. 2017; 213:271–276. PMID: 28215646.
47. Bhattacharjee B, Renzette N, Kowalik TF. Genetic analysis of cytomegalovirus in malignant gliomas. J Virol. 2012; 86:6815–6824. PMID: 22496213.
48. Ranganathan P, Clark PA, Kuo JS, Salamat MS, Kalejta RF. Significant association of multiple human cytomegalovirus genomic loci with glioblastoma multiforme samples. J Virol. 2012; 86:854–864. PMID: 22090104.
49. Matlaf LA, Harkins LE, Bezrookove V, Cobbs CS, Soroceanu L. Cytomegalovirus pp71 protein is expressed in human glioblastoma and promotes pro-angiogenic signaling by activation of stem cell factor. PLoS One. 2013; 8:e68176. PMID: 23861869.
50. dos Santos CJ, Stangherlin LM, Figueiredo EG, Corrêa C, Teixeira MJ, da Silva MC. High prevalence of HCMV and viral load in tumor tissues and peripheral blood of glioblastoma multiforme patients. J Med Virol. 2014; 86:1953–1961. PMID: 24173908.
51. Mohammad AA, Rahbar A, Lui WO, Davoudi B, Catrina A, Stragliotto G, et al. Detection of circulating hcmv-miR-UL112-3p in patients with glioblastoma, rheumatoid arthritis, diabetes mellitus and healthy controls. PLoS One. 2014; 9:e113740. PMID: 25462570.
52. Malekpour Afshar R, Mollaei HR, Zandi B, Iranpour M. Evaluation of JC and cytomegalo viruses in glioblastoma tissue. Asian Pac J Cancer Prev. 2016; 17:4907–4911. PMID: 28032494.
53. Adnan Ali SM, Mirza Y, Ahmad Z, Zahid N, Enam SA. Human papillomavirus and human cytomegalovirus infection and association with prognosis in patients with primary glioblastoma in Pakistan. World Neurosurg. 2019; 121:e931–e939. PMID: 30321676.
54. Goerig NL, Frey B, Korn K, Fleckenstein B, Überla K, Schmidt MA, et al. Early mortality of brain cancer patients and its connection to cytomegalovirus reactivation during radiochemotherapy. Clin Cancer Res. 2020; 26:3259–3270. PMID: 32060103.
55. Ghaffari H, Tavakoli A, Faranoush M, Naderi A, Kiani SJ, Sadeghipour A, et al. Molecular investigation of human cytomegalovirus and Epstein-Barr virus in glioblastoma brain tumor: a case-control study in Iran. Iran Biomed J. 2021; 25:426–433. PMID: 34696577.
56. Yuan Z, Ye X, Zhu L, Zhang N, An Z, Zheng WJ. Virome assembly and annotation in brain tissue based on next-generation sequencing. Cancer Med. 2020; 9:6776–6790. PMID: 32738030.
57. Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010; 10:878–889. PMID: 21102637.
58. Herbein G. The human cytomegalovirus, from oncomodulation to oncogenesis. Viruses. 2018; 10:408.
59. El Baba R, Herbein G. Immune landscape of CMV infection in cancer patients: from “canonical” diseases toward virus-elicited oncomodulation. Front Immunol. 2021; 12:730765. PMID: 34566995.
60. Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia. 2009; 11:1–9. PMID: 19107226.
61. Rahman M, Dastmalchi F, Karachi A, Mitchell D. The role of CMV in glioblastoma and implications for immunotherapeutic strategies. Oncoimmunology. 2018; 8:e1514921. PMID: 30546954.
62. Schuessler A, Smith C, Beagley L, Boyle GM, Rehan S, Matthews K, et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014; 74:3466–3476. PMID: 24795429.
63. Smith C, Lineburg KE, Martins JP, Ambalathingal GR, Neller MA, Morrison B, et al. Autologous CMV-specific T cells are a safe adjuvant immunotherapy for primary glioblastoma multiforme. J Clin Invest. 2020; 130:6041–6053. PMID: 32750039.
64. Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015; 519:366–369. PMID: 25762141.
65. Reap EA, Suryadevara CM, Batich KA, Sanchez-Perez L, Archer GE, Schmittling RJ, et al. Dendritic cells enhance polyfunctionality of adoptively transferred T cells that target cytomegalovirus in glioblastoma. Cancer Res. 2018; 78:256–264. PMID: 29093005.
66. Batich KA, Reap EA, Archer GE, Sanchez-Perez L, Nair SK, Schmittling RJ, et al. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin Cancer Res. 2017; 23:1898–1909. PMID: 28411277.
Table 1
Cytomegalovirus detection by immunohistochemistry
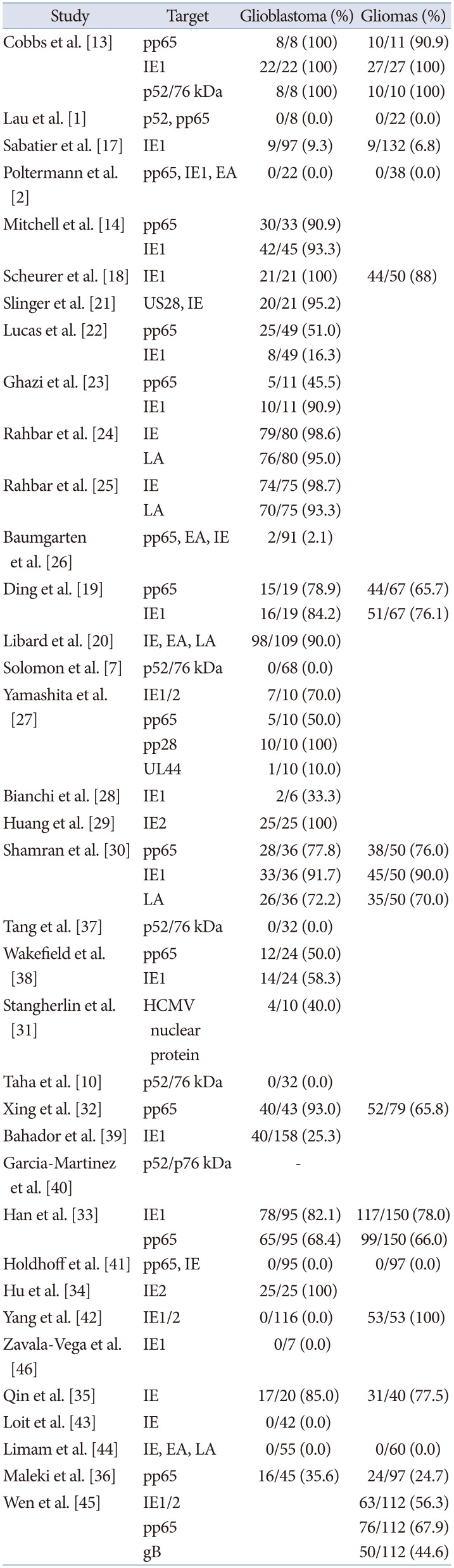
| Study | Target | Glioblastoma (%) | Gliomas (%) |
|---|---|---|---|
| Cobbs et al. [13] | pp65 | 8/8 (100) | 10/11 (90.9) |
| IE1 | 22/22 (100) | 27/27 (100) | |
| p52/76 kDa | 8/8 (100) | 10/10 (100) | |
| Lau et al. [1] | p52, pp65 | 0/8 (0.0) | 0/22 (0.0) |
| Sabatier et al. [17] | IE1 | 9/97 (9.3) | 9/132 (6.8) |
| Poltermann et al. [2] | pp65, IE1, EA | 0/22 (0.0) | 0/38 (0.0) |
| Mitchell et al. [14] | pp65 | 30/33 (90.9) | |
| IE1 | 42/45 (93.3) | ||
| Scheurer et al. [18] | IE1 | 21/21 (100) | 44/50 (88) |
| Slinger et al. [21] | US28, IE | 20/21 (95.2) | |
| Lucas et al. [22] | pp65 | 25/49 (51.0) | |
| IE1 | 8/49 (16.3) | ||
| Ghazi et al. [23] | pp65 | 5/11 (45.5) | |
| IE1 | 10/11 (90.9) | ||
| Rahbar et al. [24] | IE | 79/80 (98.6) | |
| LA | 76/80 (95.0) | ||
| Rahbar et al. [25] | IE | 74/75 (98.7) | |
| LA | 70/75 (93.3) | ||
| Baumgarten et al. [26] | pp65, EA, IE | 2/91 (2.1) | |
| Ding et al. [19] | pp65 | 15/19 (78.9) | 44/67 (65.7) |
| IE1 | 16/19 (84.2) | 51/67 (76.1) | |
| Libard et al. [20] | IE, EA, LA | 98/109 (90.0) | |
| Solomon et al. [7] | p52/76 kDa | 0/68 (0.0) | |
| Yamashita et al. [27] | IE1/2 | 7/10 (70.0) | |
| pp65 | 5/10 (50.0) | ||
| pp28 | 10/10 (100) | ||
| UL44 | 1/10 (10.0) | ||
| Bianchi et al. [28] | IE1 | 2/6 (33.3) | |
| Huang et al. [29] | IE2 | 25/25 (100) | |
| Shamran et al. [30] | pp65 | 28/36 (77.8) | 38/50 (76.0) |
| IE1 | 33/36 (91.7) | 45/50 (90.0) | |
| LA | 26/36 (72.2) | 35/50 (70.0) | |
| Tang et al. [37] | p52/76 kDa | 0/32 (0.0) | |
| Wakefield et al. [38] | pp65 | 12/24 (50.0) | |
| IE1 | 14/24 (58.3) | ||
| Stangherlin et al. [31] | HCMV nuclear protein | 4/10 (40.0) | |
| Taha et al. [10] | p52/76 kDa | 0/32 (0.0) | |
| Xing et al. [32] | pp65 | 40/43 (93.0) | 52/79 (65.8) |
| Bahador et al. [39] | IE1 | 40/158 (25.3) | |
| Garcia-Martinez et al. [40] | p52/p76 kDa | - | |
| Han et al. [33] | IE1 | 78/95 (82.1) | 117/150 (78.0) |
| pp65 | 65/95 (68.4) | 99/150 (66.0) | |
| Holdhoff et al. [41] | pp65, IE | 0/95 (0.0) | 0/97 (0.0) |
| Hu et al. [34] | IE2 | 25/25 (100) | |
| Yang et al. [42] | IE1/2 | 0/116 (0.0) | 53/53 (100) |
| Zavala-Vega et al. [46] | IE1 | 0/7 (0.0) | |
| Qin et al. [35] | IE | 17/20 (85.0) | 31/40 (77.5) |
| Loit et al. [43] | IE | 0/42 (0.0) | |
| Limam et al. [44] | IE, EA, LA | 0/55 (0.0) | 0/60 (0.0) |
| Maleki et al. [36] | pp65 | 16/45 (35.6) | 24/97 (24.7) |
| Wen et al. [45] | IE1/2 | 63/112 (56.3) | |
| pp65 | 76/112 (67.9) | ||
| gB | 50/112 (44.6) |
Table 2
Cytomegalovirus detection by in situ hybridization
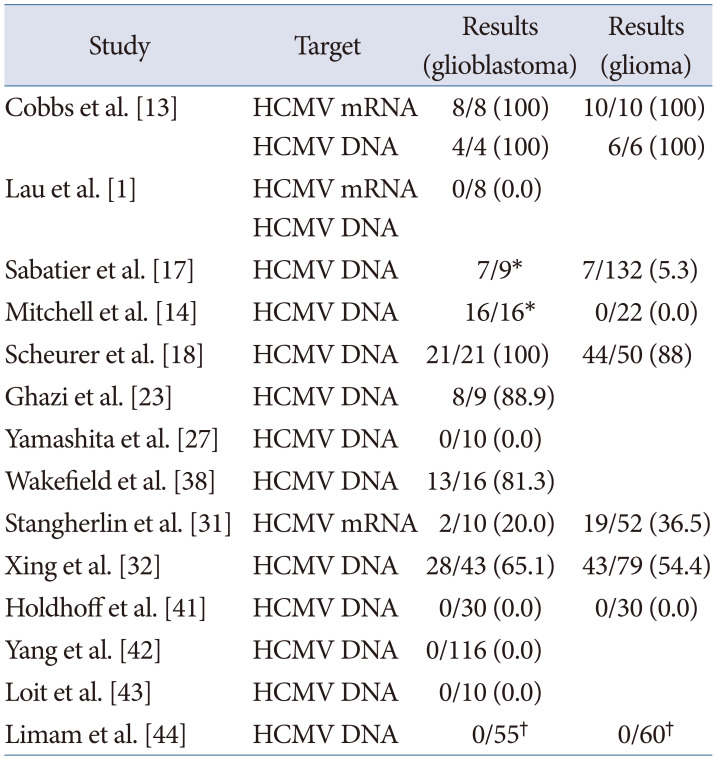
| Study | Target | Results (glioblastoma) | Results (glioma) |
|---|---|---|---|
| Cobbs et al. [13] | HCMV mRNA | 8/8 (100) | 10/10 (100) |
| HCMV DNA | 4/4 (100) | 6/6 (100) | |
| Lau et al. [1] | HCMV mRNA | 0/8 (0.0) | |
| HCMV DNA | |||
| Sabatier et al. [17] | HCMV DNA | 7/9* | 7/132 (5.3) |
| Mitchell et al. [14] | HCMV DNA | 16/16* | 0/22 (0.0) |
| Scheurer et al. [18] | HCMV DNA | 21/21 (100) | 44/50 (88) |
| Ghazi et al. [23] | HCMV DNA | 8/9 (88.9) | |
| Yamashita et al. [27] | HCMV DNA | 0/10 (0.0) | |
| Wakefield et al. [38] | HCMV DNA | 13/16 (81.3) | |
| Stangherlin et al. [31] | HCMV mRNA | 2/10 (20.0) | 19/52 (36.5) |
| Xing et al. [32] | HCMV DNA | 28/43 (65.1) | 43/79 (54.4) |
| Holdhoff et al. [41] | HCMV DNA | 0/30 (0.0) | 0/30 (0.0) |
| Yang et al. [42] | HCMV DNA | 0/116 (0.0) | |
| Loit et al. [43] | HCMV DNA | 0/10 (0.0) | |
| Limam et al. [44] | HCMV DNA | 0/55† | 0/60† |
Table 3
Cytomegalovirus detection by polymerase chain reaction
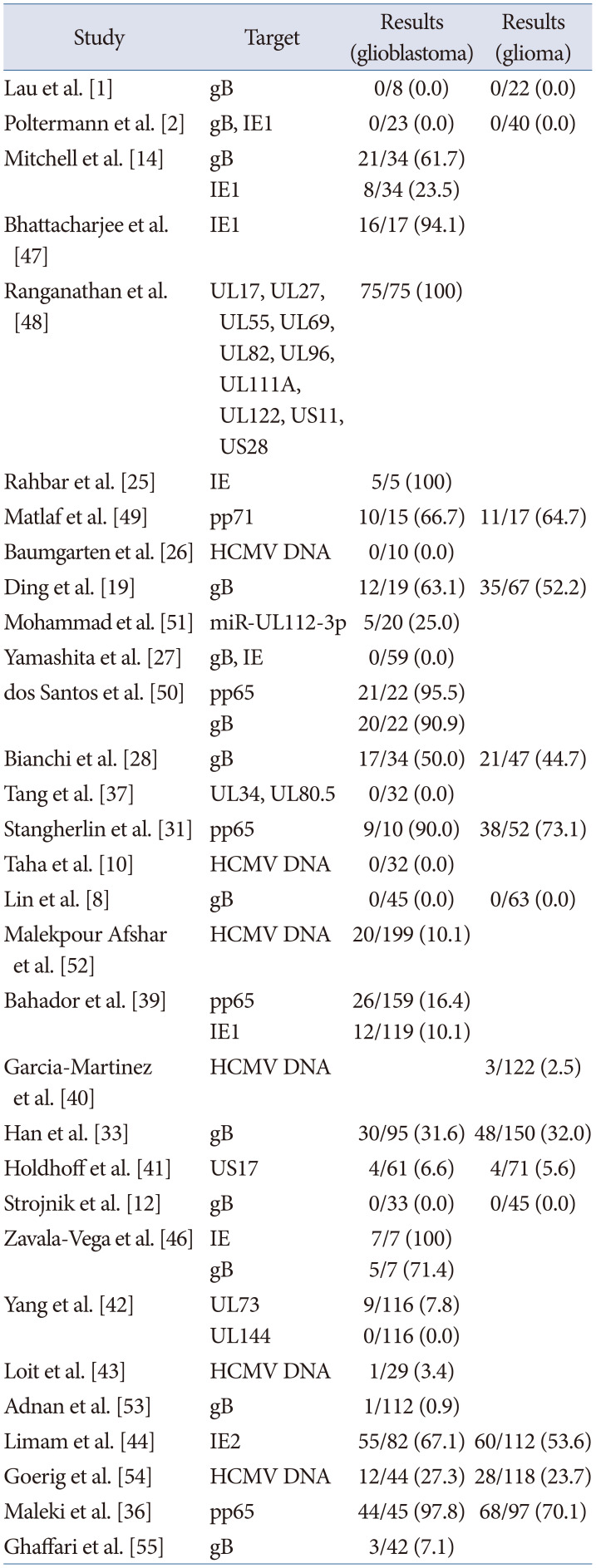
| Study | Target | Results (glioblastoma) | Results (glioma) |
|---|---|---|---|
| Lau et al. [1] | gB | 0/8 (0.0) | 0/22 (0.0) |
| Poltermann et al. [2] | gB, IE1 | 0/23 (0.0) | 0/40 (0.0) |
| Mitchell et al. [14] | gB | 21/34 (61.7) | |
| IE1 | 8/34 (23.5) | ||
| Bhattacharjee et al. [47] | IE1 | 16/17 (94.1) | |
| Ranganathan et al. [48] | UL17, UL27, UL55, UL69, UL82, UL96, UL111A, UL122, US11, US28 | 75/75 (100) | |
| Rahbar et al. [25] | IE | 5/5 (100) | |
| Matlaf et al. [49] | pp71 | 10/15 (66.7) | 11/17 (64.7) |
| Baumgarten et al. [26] | HCMV DNA | 0/10 (0.0) | |
| Ding et al. [19] | gB | 12/19 (63.1) | 35/67 (52.2) |
| Mohammad et al. [51] | miR-UL112-3p | 5/20 (25.0) | |
| Yamashita et al. [27] | gB, IE | 0/59 (0.0) | |
| dos Santos et al. [50] | pp65 | 21/22 (95.5) | |
| gB | 20/22 (90.9) | ||
| Bianchi et al. [28] | gB | 17/34 (50.0) | 21/47 (44.7) |
| Tang et al. [37] | UL34, UL80.5 | 0/32 (0.0) | |
| Stangherlin et al. [31] | pp65 | 9/10 (90.0) | 38/52 (73.1) |
| Taha et al. [10] | HCMV DNA | 0/32 (0.0) | |
| Lin et al. [8] | gB | 0/45 (0.0) | 0/63 (0.0) |
| Malekpour Afshar et al. [52] | HCMV DNA | 20/199 (10.1) | |
| Bahador et al. [39] | pp65 | 26/159 (16.4) | |
| IE1 | 12/119 (10.1) | ||
| Garcia-Martinez et al. [40] | HCMV DNA | 3/122 (2.5) | |
| Han et al. [33] | gB | 30/95 (31.6) | 48/150 (32.0) |
| Holdhoff et al. [41] | US17 | 4/61 (6.6) | 4/71 (5.6) |
| Strojnik et al. [12] | gB | 0/33 (0.0) | 0/45 (0.0) |
| Zavala-Vega et al. [46] | IE | 7/7 (100) | |
| gB | 5/7 (71.4) | ||
| Yang et al. [42] | UL73 | 9/116 (7.8) | |
| UL144 | 0/116 (0.0) | ||
| Loit et al. [43] | HCMV DNA | 1/29 (3.4) | |
| Adnan et al. [53] | gB | 1/112 (0.9) | |
| Limam et al. [44] | IE2 | 55/82 (67.1) | 60/112 (53.6) |
| Goerig et al. [54] | HCMV DNA | 12/44 (27.3) | 28/118 (23.7) |
| Maleki et al. [36] | pp65 | 44/45 (97.8) | 68/97 (70.1) |
| Ghaffari et al. [55] | gB | 3/42 (7.1) |
Table 4
Cytomegalovirus detection by next generation sequencing
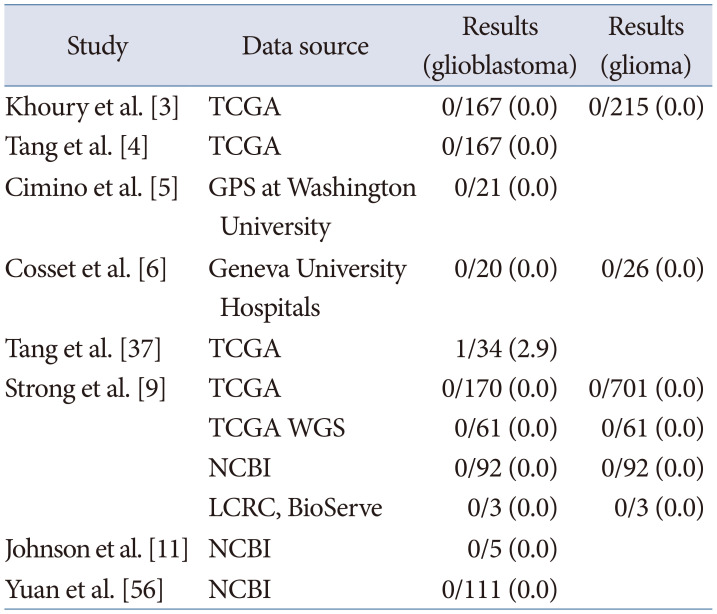
| Study | Data source | Results (glioblastoma) | Results (glioma) |
|---|---|---|---|
| Khoury et al. [3] | TCGA | 0/167 (0.0) | 0/215 (0.0) |
| Tang et al. [4] | TCGA | 0/167 (0.0) | |
| Cimino et al. [5] | GPS at Washington University | 0/21 (0.0) | |
| Cosset et al. [6] | Geneva University Hospitals | 0/20 (0.0) | 0/26 (0.0) |
| Tang et al. [37] | TCGA | 1/34 (2.9) | |
| Strong et al. [9] | TCGA | 0/170 (0.0) | 0/701 (0.0) |
| TCGA WGS | 0/61 (0.0) | 0/61 (0.0) | |
| NCBI | 0/92 (0.0) | 0/92 (0.0) | |
| LCRC, BioServe | 0/3 (0.0) | 0/3 (0.0) | |
| Johnson et al. [11] | NCBI | 0/5 (0.0) | |
| Yuan et al. [56] | NCBI | 0/111 (0.0) |
Table 5
Published clinical trials of CMV-specific immunotherapy for glioblastoma
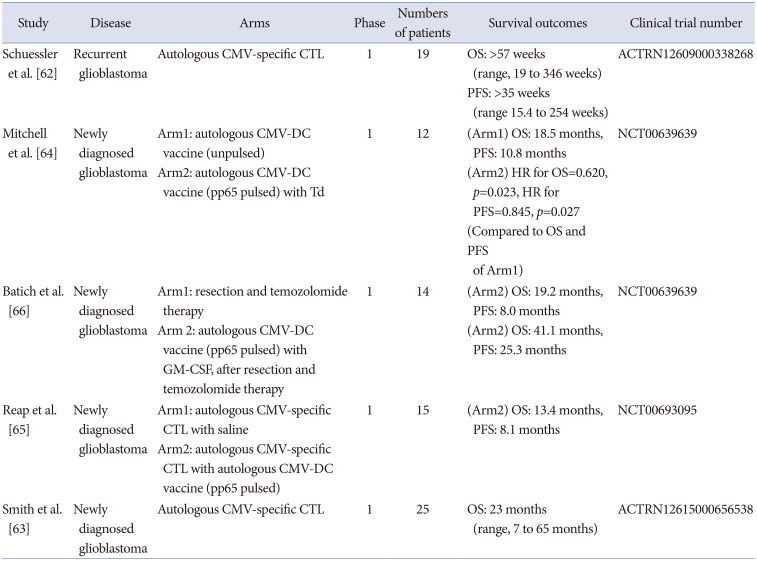
| Study | Disease | Arms | Phase | Numbers of patients | Survival outcomes | Clinical trial number |
|---|---|---|---|---|---|---|
| Schuessler et al. [62] | Recurrent glioblastoma | Autologous CMV-specific CTL | 1 | 19 |
OS: >57 weeks (range, 19 to 346 weeks) PFS: >35 weeks (range 15.4 to 254 weeks) |
ACTRN12609000338268 |
| Mitchell et al. [64] | Newly diagnosed glioblastoma |
Arm1: autologous CMV-DC vaccine (unpulsed) Arm2: autologous CMV-DC vaccine (pp65 pulsed) with Td |
1 | 12 |
(Arm1) OS: 18.5 months, PFS: 10.8 months (Arm2) HR for OS=0.620, p=0.023, HR for PFS=0.845, p=0.027 (Compared to OS and PFS of Arm1) |
NCT00639639 |
| Batich et al. [66] | Newly diagnosed glioblastoma |
Arm1: resection and temozolomide therapy Arm 2: autologous CMV-DC vaccine (pp65 pulsed) with GM-CSF, after resection and temozolomide therapy |
1 | 14 |
(Arm2) OS: 19.2 months, PFS: 8.0 months (Arm2) OS: 41.1 months, PFS: 25.3 months |
NCT00639639 |
| Reap et al. [65] | Newly diagnosed glioblastoma |
Arm1: autologous CMV-specific CTL with saline Arm2: autologous CMV-specific CTL with autologous CMV-DC vaccine (pp65 pulsed) |
1 | 15 | (Arm2) OS: 13.4 months, PFS: 8.1 months | NCT00693095 |
| Smith et al. [63] | Newly diagnosed glioblastoma | Autologous CMV-specific CTL | 1 | 25 | OS: 23 months (range, 7 to 65 months) | ACTRN12615000656538 |
Table 6
Unpublished clinical trials of CMV-specific immunotherapy for glioblastoma
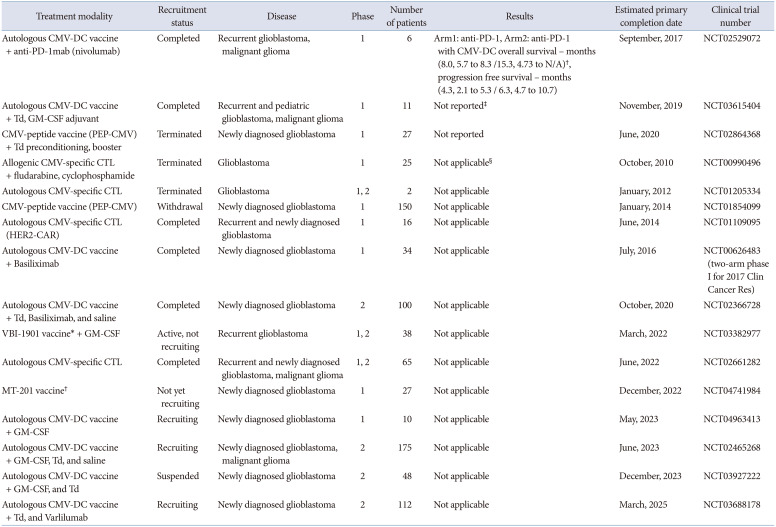
| Treatment modality | Recruitment status | Disease | Phase | Number of patients | Results | Estimated primary completion date | Clinical trial number |
|---|---|---|---|---|---|---|---|
| Autologous CMV-DC vaccine + anti-PD-1mab (nivolumab) | Completed | Recurrent glioblastoma, malignant glioma | 1 | 6 | Arm1: anti-PD-1, Arm2: anti-PD-1 with CMV-DC overall survival – months (8.0, 5.7 to 8.3 /15.3, 4.73 to N/A)†, progression free survival – months (4.3, 2.1 to 5.3 / 6.3, 4.7 to 10.7) | September, 2017 | NCT02529072 |
| Autologous CMV-DC vaccine + Td, GM-CSF adjuvant | Completed | Recurrent and pediatric glioblastoma, malignant glioma | 1 | 11 | Not reported‡ | November, 2019 | NCT03615404 |
| CMV-peptide vaccine (PEP-CMV) + Td preconditioning, booster | Terminated | Newly diagnosed glioblastoma | 1 | 27 | Not reported | June, 2020 | NCT02864368 |
| Allogenic CMV-specific CTL + fludarabine, cyclophosphamide | Terminated | Glioblastoma | 1 | 25 | Not applicable§ | October, 2010 | NCT00990496 |
| Autologous CMV-specific CTL | Terminated | Glioblastoma | 1, 2 | 2 | Not applicable | January, 2012 | NCT01205334 |
| CMV-peptide vaccine (PEP-CMV) | Withdrawal | Newly diagnosed glioblastoma | 1 | 150 | Not applicable | January, 2014 | NCT01854099 |
| Autologous CMV-specific CTL (HER2-CAR) | Completed | Recurrent and newly diagnosed glioblastoma | 1 | 16 | Not applicable | June, 2014 | NCT01109095 |
| Autologous CMV-DC vaccine + Basiliximab | Completed | Newly diagnosed glioblastoma | 1 | 34 | Not applicable | July, 2016 | NCT00626483 (two-arm phase I for 2017 Clin Cancer Res) |
| Autologous CMV-DC vaccine + Td, Basiliximab, and saline | Completed | Newly diagnosed glioblastoma | 2 | 100 | Not applicable | October, 2020 | NCT02366728 |
| VBI-1901 vaccine* + GM-CSF | Active, not recruiting | Recurrent glioblastoma | 1, 2 | 38 | Not applicable | March, 2022 | NCT03382977 |
| Autologous CMV-specific CTL | Completed | Recurrent and newly diagnosed glioblastoma, malignant glioma | 1, 2 | 65 | Not applicable | June, 2022 | NCT02661282 |
| MT-201 vaccine† | Not yet recruiting | Newly diagnosed glioblastoma | 1 | 27 | Not applicable | December, 2022 | NCT04741984 |
| Autologous CMV-DC vaccine + GM-CSF | Recruiting | Newly diagnosed glioblastoma | 1 | 10 | Not applicable | May, 2023 | NCT04963413 |
| Autologous CMV-DC vaccine + GM-CSF, Td, and saline | Recruiting | Newly diagnosed glioblastoma, malignant glioma | 2 | 175 | Not applicable | June, 2023 | NCT02465268 |
| Autologous CMV-DC vaccine + GM-CSF, and Td | Suspended | Newly diagnosed glioblastoma | 2 | 48 | Not applicable | December, 2023 | NCT03927222 |
| Autologous CMV-DC vaccine + Td, and Varlilumab | Recruiting | Newly diagnosed glioblastoma | 2 | 112 | Not applicable | March, 2025 | NCT03688178 |
*VBI-1901 vaccine refers to an enveloped virus-like particle vaccine targeting CMV antigens, gB and pp65; †MT-201 vaccine refers to a pp65 monocyte vaccine in which monocytes are isolated from patients’ leukapheresis. CMV, cytomegalovirus; DC, dendritic cell; CTL, cytotoxic T lymphocyte; GM-CSF, granulocyte macrophage-colony stimulating factor; Td, tetanus and diphtheria toxoid; CAR, chimeric antigen receptor; HER-2, human epidermal growth factor receptor 2; PD-1, programmed cell death protein 1




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download