1. Feindel W. Osler vindicated: glioma of the leg center with Jacksonian epilepsy; removal and cure, with a 50-year follow-up. Historical vignette. J Neurosurg. 2009; 111:293–300. PMID:
19267535.
2. Daumas-Duport C, Scheithauer BW, Chodkiewicz JP, Laws ER Jr, Vedrenne C. Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizures. Report of thirty-nine cases. Neurosurgery. 1988; 23:545–556. PMID:
3143922.
3. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23:1231–1251. PMID:
34185076.
4. Scheithauer BW. Development of the WHO classification of tumors of the central nervous system: a historical perspective. Brain Pathol. 2009; 19:551–564. PMID:
18771526.
5. Dho YS, Jung KW, Ha J, Seo Y, Park CK, Won YJ, et al. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 2017; 5:16–23. PMID:
28516074.
6. Cho KT, Wang KC, Kim SK, Shin SH, Chi JG, Cho BK. Pediatric brain tumors: statistics of SNUH, Korea (1959-2000). Childs Nerv Syst. 2002; 18:30–37. PMID:
11935241.
7. Luyken C, Blümcke I, Fimmers R, Urbach H, Elger CE, Wiestler OD, et al. The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003; 44:822–830. PMID:
12790896.
8. Slegers RJ, Blumcke I. Low-grade developmental and epilepsy associated brain tumors: a critical update 2020. Acta Neuropathol Commun. 2020; 8:27. PMID:
32151273.
9. Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med. 2017; 377:1648–1656. PMID:
29069555.
10. Campos AR, Clusmann H, von Lehe M, Niehusmann P, Becker AJ, Schramm J, et al. Simple and complex dysembryoplastic neuroepithelial tumors (DNT) variants: clinical profile, MRI, and histopathology. Neuroradiology. 2009; 51:433–443. PMID:
19242688.
11. Daumas-Duport C. Dysembryoplastic neuroepithelial tumours. Brain Pathol. 1993; 3:283–295. PMID:
8293188.
12. Sakuta R, Otsubo H, Nolan MA, Weiss SK, Hawkins C, Rutka JT, et al. Recurrent intractable seizures in children with cortical dysplasia adjacent to dysembryoplastic neuroepithelial tumor. J Child Neurol. 2005; 20:377–384. PMID:
15921242.
13. Chassoux F, Landré E, Mellerio C, Laschet J, Devaux B, Daumas-Duport C. Dysembryoplastic neuroepithelial tumors: epileptogenicity related to histologic subtypes. Clin Neurophysiol. 2013; 124:1068–1078. PMID:
23276492.
14. Urbach H. MRI of long-term epilepsy-associated tumors. Semin Ultrasound CT MR. 2008; 29:40–46. PMID:
18383906.
15. Phi JH, Paeng JC, Lee HS, Wang KC, Cho BK, Lee JY, et al. Evaluation of focal cortical dysplasia and mixed neuronal and glial tumors in pediatric epilepsy patients using 18F-FDG and 11C-methionine pet. J Nucl Med. 2010; 51:728–734. PMID:
20395328.
16. Chassoux F, Rodrigo S, Mellerio C, Landré E, Miquel C, Turak B, et al. Dysembryoplastic neuroepithelial tumors: an MRI-based scheme for epilepsy surgery. Neurology. 2012; 79:1699–1707. PMID:
23035071.
17. Yang J, Kim SK, Kim KJ, Chae JH, Lim BC, Wang KC, et al. Satellite lesions of DNET: implications for seizure and tumor control after resection. J Neurooncol. 2019; 143:437–445. PMID:
31054098.
18. Piertsch T, Ellison D, Hirose T, Jacques T, Schuller U, Varlet P. Dysembryoplastic neuroepithelial tumour. WHO Classification of Tumours Editorial Board. Central Nervous System Tumors. 5th ed. Lyon: Internatioal Agency for Research on Cancer;2021. p. 123–126.
19. Rivera B, Gayden T, Carrot-Zhang J, Nadaf J, Boshari T, Faury D, et al. Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol. 2016; 131:847–863. PMID:
26920151.
20. Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016; 131:833–845. PMID:
26810070.
21. Chuang NA, Yoon JM, Newbury RO, Crawford JR. Glioblastoma multiforme arising from dysembryoplastic neuroepithelial tumor in a child in the absence of therapy. J Pediatr Hematol Oncol. 2014; 36:e536–e539. PMID:
24309599.
22. Heiland DH, Staszewski O, Hirsch M, Masalha W, Franco P, Grauvogel J, et al. Malignant transformation of a dysembryoplastic neuroepithelial tumor (DNET) characterized by genome-wide methylation analysis. J Neuropathol Exp Neurol. 2016; 75:358–365. PMID:
26921879.
23. Maher CO, White JB, Scheithauer BW, Raffel C. Recurrence of dysembryoplastic neuroepithelial tumor following resection. Pediatr Neurosurg. 2008; 44:333–336. PMID:
18552517.
24. Qaddoumi I, Ellison DW, Morris EB, Broniscer A, Boop F, Merchant T, et al. Dysembryoplastic neuroepithelial tumors and cognitive outcome: cure at a price? Cancer. 2010; 116:5461–5469. PMID:
20672357.
25. Bonney PA, Boettcher LB, Conner AK, Glenn CA, Briggs RG, Santucci JA, et al. Review of seizure outcomes after surgical resection of dysembryoplastic neuroepithelial tumors. J Neurooncol. 2016; 126:1–10. PMID:
26514362.
26. Giulioni M, Rubboli G, Marucci G, Martinoni M, Volpi L, Michelucci R, et al. Seizure outcome of epilepsy surgery in focal epilepsies associated with temporomesial glioneuronal tumors: lesionectomy compared with tailored resection. J Neurosurg. 2009; 111:1275–1282. PMID:
19408976.
27. Tandon N, Esquenazi Y. Resection strategies in tumoral epilepsy: is a lesionectomy enough? Epilepsia. 2013; 54 Suppl 9:72–78.
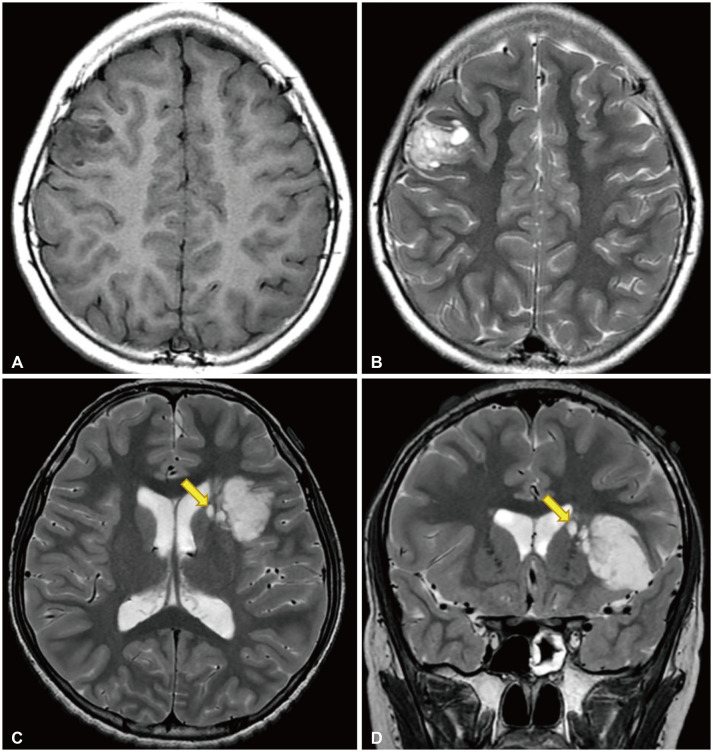
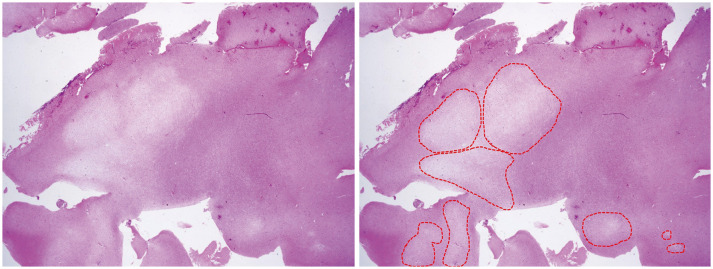
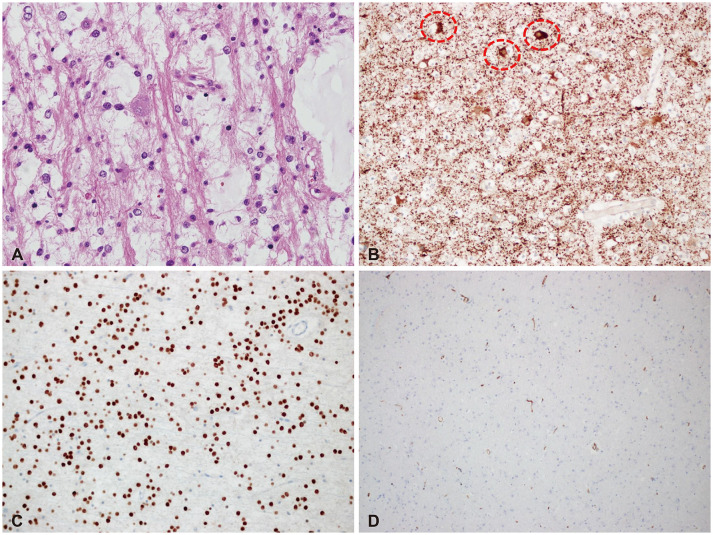
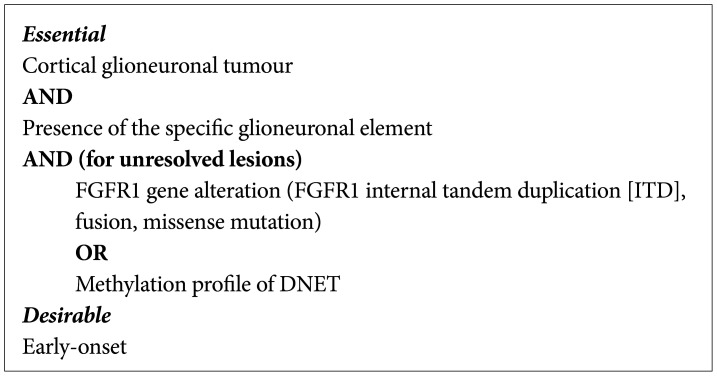




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download