DISCUSSION
IgG4-RD is a chronic immune-mediated fibro-inflammatory condition with systemic infiltration of multiple organs. The pathologic hallmarks of IgG4-RD are dense lymphoplasmacytic infiltrations with a predominance of IgG4 plasma cells in the affected tissue with storiform fibrosis and obliterative phlebitis [
1]. The number of IgG4-positive plasma cells per HPF ranging from minimum of 30 to 50 is regarded as a sufficient criterion for the diagnosis of IgG4-RD [
4]. Elevated levels of serum IgG4 is frequent in the acute phase up to 51%–70% [
5], and patients usually have a good recovery after being treated with systemic glucocorticosteroid. Any organs can be involved, but the most commonly involved organs are the pancreas, kidneys, orbital adnexal structures, salivary glands, and retroperitoneum [
6]. Due to its specific histopathological features and tumor-like swelling of involved organs, IgG4-RD affected patients are often misdiagnosed as having a malignancy since the lesions can mimic tumors, infections, or immune-mediated disease [
7].
Their intracranial presentation is relatively rare, although various manifestations are recognized. There are published descriptions of hypertrophic pachymeningitis, inflammatory lesions of the pituitary gland, inflammatory orbital pseudotumor, pterygopalatine fossa, and cranial nerve involvement in IgG4-RD [
8]. Among them, hypertrophic pachymeningitis and hypophysitis are the most frequent manifestations [
2]. The first report of intracranial involvement by IgG4-RD occurred in the context of hypophysitis [
9]. Since then, hypertrophic pachymeningitis has also been recognized to occur as part of the IgG4-RD spectrum, and IgG4-RD may account for a substantial percentage of cases previously regarded as idiopathic [
10].
The pathogenesis of IgG4-RD is still under debate, yet there is growing evidence that it has an autoimmune basis, with important roles for both B and T cells, especially CD4+ and T-follicular helper cells. CD4+ T cells, the most abundant cells within affected tissues, are dispersed throughout IgG4-RD lesions [
11]. These cytotoxic T cells make products such as granzyme B, perforin, IL-1, transforming growth factor-beta, and interferon-gamma that are potentially important mediators of the fibrosis which is a dominant part of histopathology in IgG4-RD [
11]. A T-follicular helper cell response that is separate from the CD4+ cytotoxic T lymphocytes is likely to be responsible for the development of germinal centers within lymph nodes (and involved organs) and the production of cytokines that drive the IgG4 class-switch, culminating in the creation of IgG4-secreting plasmablasts and long-lived plasma cells [
12]. Taken together, it is suggested that CD4+ cytotoxic T cells play a central role in the disease and that they are sustained by continuous antigen presentation by cells of the B lymphocyte lineage, including but not limited to plasmablasts [
11]. Recent consensus is that the IgG4 antibodies in this disease are not pathogenic themselves, but rather represent an epiphenomenon, possibly having an anti-inflammatory role.
As discussed earlier, because IgG4-RD’s extensive infiltration tends to form a mass-like lesion, it is often confused with neoplasms. Despite its mass-forming characteristics, the specific histopathologic features of IgG4-RD are distinct from that of neoplasms. However, there have been increasing studies that reported a spectrum of malignancies occurring in association with IgG4-RD, including solid tumors located in the lung, pancreas, gastrointestinal tract, and prostate, as well as lymphoma [
1314151617]. Tang et al. [
18] reported in a cohort study that IgG4-RD patients have a 2.78-fold increased risk of having malignancy. The relationships between these two diseases remain unclear and are the subject of on-going research.
Although the mechanism underlying the co-occurrence of IgG4-RD and malignancies has not been fully determined to date, there have been numerous suggestive hypothesis. It is thought to be established that long-standing chronic inflammation plays a critical role in the development of cancer through the process of inflammation-associated carcinogenesis [
19]. It could be because IgG4-RD causes sustained inflammation that may induce preferable cancerous microenvironments. Some suggests that elevated IgG4 and extensive lymphoplasmacytic inflammation shown in IgG4-RD might be an evoked response caused by antigenicity of initial cancer [
14].
Other than the relationship between the malignancies and IgG4-RD, it is noteworthy that various kinds of malignancies show positive correlation with elevated IgG4 itself. IgG4 responses have also been reported in different cancers such as melanoma [
202122], extrahepatic cholangiocarcinoma [
23], pancreatic cancer [
24], and glioblastoma [
25]. IgG4 is usually the least-represented IgG subclass in human serum, comprising less than 4% of the total IgG. High IgG4 levels usually occur following repeated or chronic exposure to an antigen, and are associated with inflammation in a range of chronic pathological conditions [
26]. Elevated levels of IgG4 are also associated with immune tolerance under conditions of chronic exposure to a specific antigen. IgG4 was found to positively correlate with regulatory T-cells and to negatively correlate with cytotoxic T lymphocytes, supporting its involvement to immune tolerance [
27]. If tumor lesions are thought of as pathological conditions which resemble chronic inflammation, IgG4 elevations may be a response triggered by antigenicity in tumor microenvironment [
28]. Because there is yet a lack of evidence for tumor-associated IgG4 antibodies, it seems that IgG4 elevation in several types of malignancies may be a nonspecific secondary response for immune tolerance, rather than a specific response for tumor-associated antigen. Crescioli et al. [
28] inferred that additional research should be required to ascertain the tumor reactivity and antigen specificity of tumor-associated IgG4 in patients with cancer.
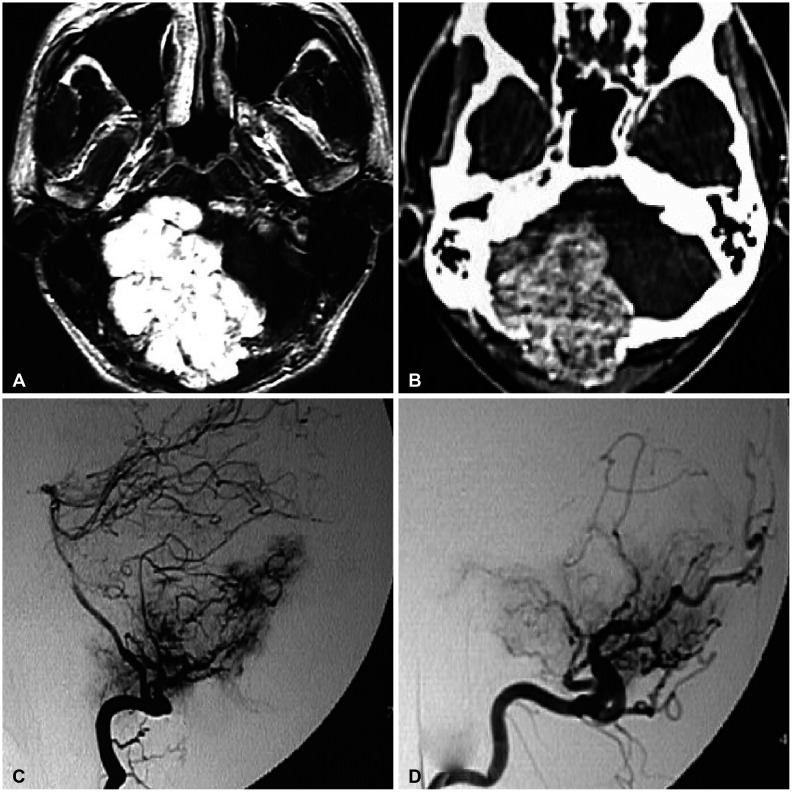
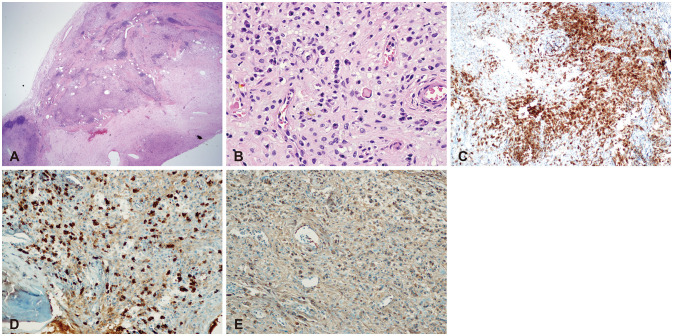
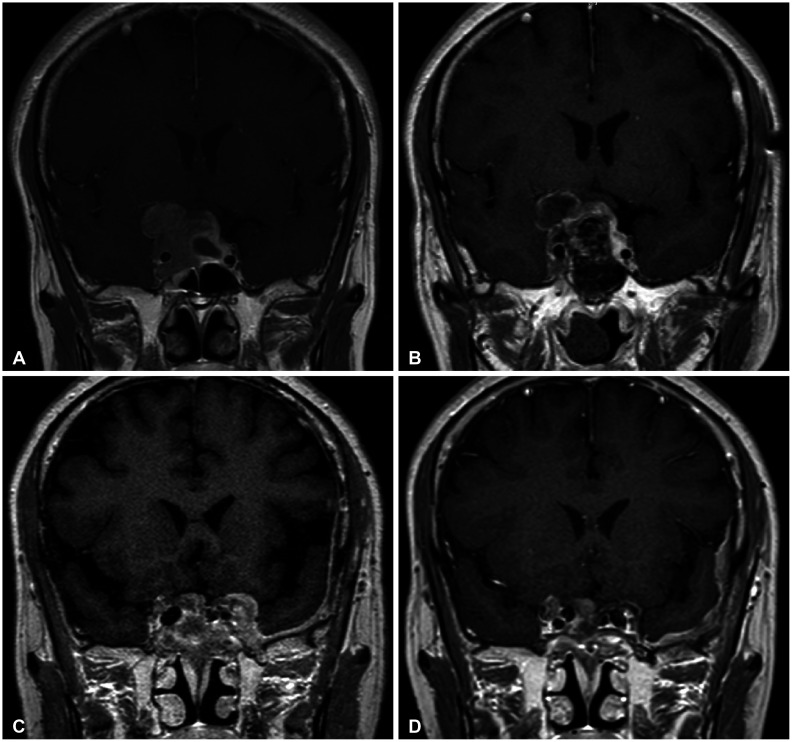
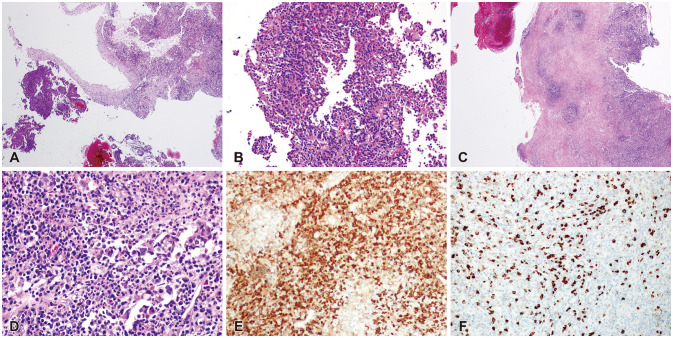
There yet has been no literature that reported the co-existence or sequential occurrence of IgG4-RD with true intracranial tumors, nor any that described pathologically confirmed IgG4-positive cells within the tumor. We hereby report two cases with a meningioma and a pituitary adenoma strongly associated with IgG4-related condition. Interestingly, their origins of tumors; meninges and the pituitary gland, are the most common intracranial sites that IgG4-RD infiltrates. Because both cases are benign tumors, their pathogenesis may not necessarily depict that of other IgG4-related malignancies as described above. According to recent literature, systemic serum IgG4 elevation in IgG4-RD or some types of malignancies seem to be a secondary epiphenomenon rather than IgG4 antibodies being pathogenic themselves [
2829]. However, it is noteworthy that in our cases the regional tumors were directly infiltrated by IgG4-positive inflammatory cells. This onsite direct interaction between inflammatory cells and tumor of their origins may be different from the hypothesized pathogenesis of various malignancies associated with IgG4-RD. Also, the benign nature of the two cases is somewhat eccentric compared to the known literature, indicating a need for different description of their pathogenesis.
We implicate from these unorthodox feature of our cases that further investigation of IgG4 immunology should be conducted. This could help us better understand the pathogenesis of IgG4-RD and lymphocytic tumor, and also lead us to a better understanding of the pathogenesis of IgG4-RD and also tumor antigenicity.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download