Abstract
Pituicytoma is a rare solid benign tumor of the sellar and/or suprasellar region originating from the pituicytes of the neurohypophysis or infundibulum, which is not differentiated from a pituitary adenoma that is diagnosed mostly in the sellar and/or suprasellar region. In addition, cystic tumors are very rare and have not been reported due to their solid and hypervascular natures. A 33-year-old man presented with a chronic headache which exacerbated recently. MRI was performed and revealed a cystic tumor in the sellar and suprasellar regions with a small parenchymal island in the cyst compressing the optic chiasm. The endoscopic endonasal transsphenoidal approach was used to remove the tumor. Immunohistochemical staining was positive for thyroid transcription factor 1, S-100 protein, and glial fibrillary acidic protein. The pituicytoma was diagnosed based on histologic findings. The authors review herein the literature on clinical presentation, diagnosis, surgical management, and outcome.
Pituicytoma is classified as a World Health Organization (WHO) grade I, rare, spindle cell glial tumor in adults, originating from the neurohypophysis or infundibulum, and most likely developing from the specialized glial cells of the region [1]. Pituicytomas show a compact architecture with interlacing fascicles of bipolar glial cells and no Rosenthal fibers, which is important for the differential diagnosis of a pilocytic astrocytoma [1].
Pituicytoma is an entity whose diagnosis and therapeutic approach has not been established due to its low frequency and scarce literature, represented mainly by case reports [2].
It is clinically challenging to differentiate a pituicytoma from other neoplasms in the sellar and suprasellar regions. In addition, there are no specific endocrinological findings and radiological images of these tumors. The diagnosis of the tumor is based on pathological examination with immunohistochemical positivity, including thyroid transcription factor 1 (TTF-1) [3].
Pituicytoma is a benign solid tumor histologically, but its hypervascularity is an obstacle to removing it surgically and can lead to a second-stage operation. Local recurrence following subtotal resection is frequent [4]. Radiologic images of pituicytomas have revealed infrequent small cystic changes with its portion existing in the degenerative area of the tumor tissue [5].
We report the case of a pure cystic pituicytoma and review the clinical, radiological, and surgical features of the tumor with relevant literature.
A 33-year-old foreign soldier presented to our clinic with a history of intermittent headaches occurring over a year, which was exacerbated as to disturb his military life recently following his experience like the thunderclap.
There were no neurologic symptoms or signs on physical examination.
MRI revealed a cystic lesion at the sellar region with some parenchymal isointensity areas in the cyst compressing the optic chiasm that outpouched into the suprasellar area. The cyst wall was enhanced with gadolinium contrast medium, but the parenchyma mass in the cyst was isointense or slightly hyperintense (Fig. 1).
Endocrinological laboratory findings were within normal ranges, including the anterior pituitary hormone studies, except for low thyroid stimulating hormone (TSH) and testosterone levels, which were restored postoperatively. There was no diabetes insipidus.
The operation was performed using an endoscopic transsphenoidal approach. After opening the sellar floor, a yellow capsule and clear, straw-colored fluid were emitted. A pulsating cyst capsule was observed in the cavity. The cyst capsule was biopsied with a punch, and curetted through the cavity internally and removed. Still, total removal was difficult because the cyst was tightly adhered to the surrounding pituitary tissue.
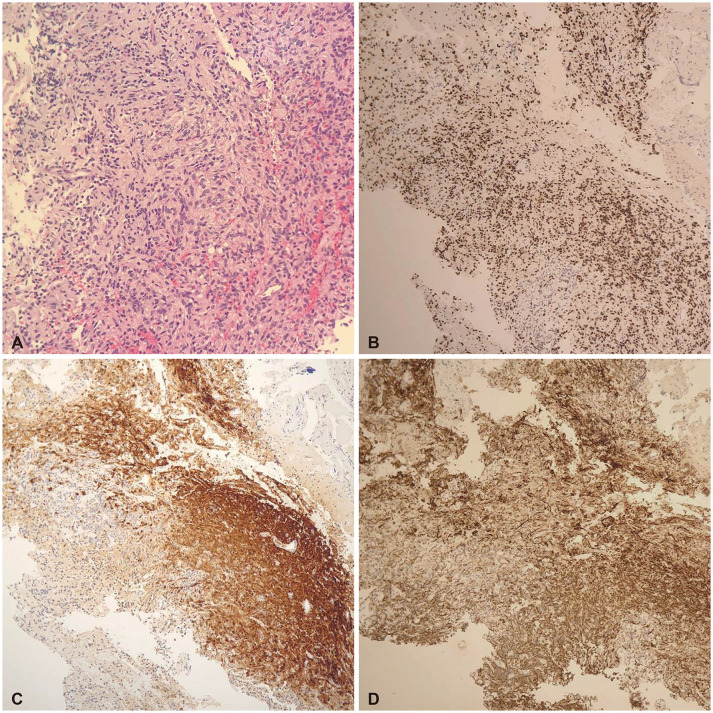
Pathologic diagnosis revealed a pituicytoma, in which tumor cells were arranged in sheets and fascicles and were spindled to epithelioid with abnormal eosinophilic cytoplasm in histologic findings. Immunohistochemical staining was positive for S-100, TTF-1, and glial fibrillary acidic protein (GFAP), and mildly positive for synaptophysin; however, reticulin staining was not informative (Fig. 2).
Postoperatively, the patient had polyuria with a urine specific gravity of 1.000 to 1.003 for 3 days, but after that, the urine output normalized with a urine specific gravity of 1.006 to 1.010. Postoperative endocrinological studies were within normal ranges. A week later, the patient returned to his country.
Liss and Kahn [6] first reported the term “pituicytoma” in 1958. They described pituicytes in 4 groups, noting that the cellular elements of the neurohypophysis were modified glial elements. However, the glioma of the posterior pituitary gland and stalk was previously reported by Antoni in 1950 [7] and Scothorne in 1955 [8].
Historically, various terms were used to describe “pituicytoma” in the region of the sellar turcica, such as infundibuloma, granular cell myoblastoma, choristoma, and granular cell tumor nosologically [9].
Brat et al. [10] formally defined pituicytoma in the year 2000 as a distinctive, low-grade spindle cell glioma that originates from pituicytes, which are specialized glia that have a sustentacular function in the neurohypophysis.
Pituicytoma was updated and listed in the WHO classification of tumors of the central nervous system, published in 2007 and 2016, respectively [11].
A novel aspect in the 4th edition of the WHO classification of endocrine tumors released in 2017 is the addition of nonendocrine tumors of the posterior pituitary, which are a distinct group of low-grade neoplasms of the sellar region. These tumors include pituicytomas, granular cell tumors of the sellar region, spindle cell oncocytomas, and sellar ependymomas, as the morphological spectrum of a single nosological entity with positive TTF-1 [12]. This is a significant change in the classification of posterior pituitary tumors in the sellar region.
Pituicytomas and nontumorous pituicytes consistently expressed staining for TTF-1, which is a histogenetic marker as mentioned above.
TTF-1, a specific marker for pulmonary and thyroid tissues, is also expressed and is required for the normal development of the ventral forebrain, from which adult pituicytes and the pituitary gland develop [3].
The immunohistochemical reactivity for pituicytomas also localizes the S-100 protein, vimentin, and GFAP [13].
The pituicytoma is solid and composed of densely arranged spindle or oblong cells with vague boundaries, circular nuclei, sparse chromatin, and a small nucleolus. The authors did not observe microcysts, Rosenthal fibers, eosinophilic granular bodies, endothelial hyperplasia, or necrosis. Mitotic figures have been reported to be rare [14]. In the present study, tumor cells were arranged in sheets and fascicles and were spindled to epithelioid with abnormal eosinophilic cytoplasm in histological findings. Immunohistochemical staining was positive for S-100, TTF-1, and GFAP and weakly positive for synaptophysin, but reticulin staining was not informative (Fig. 2).
Pituicytomas are benign, hormonally inactive tumors in the sellar and/or suprasellar region that should be differentiated from tumors originating from the pituitary gland, such as pituitary adenomas, craniopharyngiomas, meningiomas, and pilocytic astrocytomas. These tumors have similar clinical symptoms and signs, endocrinological features, and radiological imaging findings [14].
The incidence of pituicytoma is unknown due to its scarcity, and most of the literature exists as case reports and small case series. However, Shibuya [12] reported an incidence of less than 0.5% of sellar tumors. Currently, Salge-Arrieta et al. [9] published a retrospective review of case reports to date of 117 cases. The most prevalent age was the middle age between 40 to 60 years old, and it had a slightly male preponderance of 58.3%.
The progressive and chronic clinical symptoms vary according to tumor size and location. The most frequent complaint is visual field impairment, followed by endocrine disturbances, headache, and fatigue [15]. Our patient complained of a chronic headache without any other visual impairment. Endocrine alterations were anterior pituitary hormone deficit with a mild elevation of prolactin level, secondary to compression of the pituitary stalk and sexual dysfunction. Despite the location in the posterior pituitary lobe, pituicytomas are rarely associated with diabetes insipidus, which is explained by the fact that the cell bodies of vasopressin-secreting neurons remain intact in the hypothalamus [9].
In our case, the preoperative endocrine function revealed normal ranges, except for lower levels of TSH and testosterone.
Pituicytomas have been described as solid masses in sellar and/or suprasellar regions that tend to be well-circumscribed in oval or round shapes. Most of the reported MRI findings show isointensities in 86% of T1 weighted images with few hyperintensities, and hypointense or slightly hyperintense on T2 weighted images. Gadolinium contrast MRI scans have revealed substantial homogenous enhancements (74%), although some had heterogeneous enhancements (26%). These enhanced tumors were highly vascular [16,17]. The angiogram revealed a significant vascular blush [18].
On this presentation, the cystic tumor containing small isointense parenchyma was radiologically located in the sellar and suprasellar region compressing the optic chiasm on MRI findings such that clinically visual impairment was not noticed. The cystic wall was moderately enhanced.
In review of over 70 reports for pituicytomas, the whole cystic tumor such as the presented case was not mentioned. Seventeen cases of 117 pituicytomas described only the small portion in the solid tumor parenchyma with low density in CT brain scan or hypointensity in brain MRI images and which most likely occurred from the degenerative change in the tumoral parenchyma [5]. In addition, it may be mentioned that intratumoral bleedings or apoplexy are another explanations.
Considering this, the apoplexy causing the cyst formation was most likely due to the hemorrhagic transformation or to the intralesional ischemia secondary to the rupture or the occlusion of supplying vessels [19,20].
A study reported surgical resection in 108 of 117 cases either through craniotomy or the transsphenoidal approaches [9]. According to the literature review, pituicytomas are reported to be more vascular than other suprasellar tumors. This vascularity causes massive bleeding during the surgery. This is presumably the main reason for the subtotal resection of tumors with tight attachment to the infundibulum or posterior pituitary gland. Second-stage procedures were performed in 6 cases due to heavy hemorrhaging from the highly vascular tumor during the initial surgery. Strong enhancement with gadolinium contrast for preoperative embolization was necessary before the operation to prevent the hemorrhage [21].
The endoscopic endonasal transsphenoidal approach was performed in our patient with partial removal, due to the tightly adhered cyst wall to the surrounding tissues.
Prognosis in the reported series is not favorable, with a high morbidity rate (59%); however, there are no reports of highly-incapacitated patients, and operative procedures yield low mortality rates. Complications include hypopituitarism, visual impairment, and diabetes insipidus in the postoperative course. Diabetes insipidus developed postoperatively in 9 cases among 117 cases [9].
Neither malignant transformation nor metastasis from a pituicytoma has been reported. Our patient recovered within a week postoperatively including normalization of of TSH level and testosterone. Temporary polyuria with low urine specific gravity has been observed for 3 days postoperatively [12,15].
In conclusion, the presented cystic pituicytoma is a rare and unusual cystic case of a reported pituicytoma containing the smallest lesion in which apoplexy or degenerative changes take place in the parenchyma. Preoperative diagnosis can be differentiated from other cystic tumors in the suprasellar region based on the clinical symptoms and radiological images.
Notes
Author Contributions:
Conceptualization: Young Soo Ha.
Data curation: Young Soo Ha, Soo Eun Lee.
Formal analysis: Young Soo Ha, Sung Choon Park.
Funding acquisition: Young Soo Ha.
Investigation: Young Soo Ha, Yoon Yang Jung.
Methodology: Young Soo Ha.
Project administration: Young Soo Ha, Sung Yeol Ahn.
Resources: Young Soo Ha.
Visualization: Young Soo Ha.
Writing—original draft: Yoiung Soo Ha.
Writing—review & editing: Young Soo Ha.
Ethics Statement
The institutional review board (IRB) exempted informed consent due to its retrospective nature and minimal risk for harm to the patient, and this report was conducted according to the guidelines of the Declaration of Helsinki for biomedical research.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Fuller GN, Ribalta T, Wildrick DM. Brain tumors: an overview of current histopathologic and genentic classification. Winn HR, editor. Youmans and Winn neurological surgery. 7th ed. Vol 2. Philadelphia: Elsevier;2017. p. 774.
2. Viaene AN, Lee EB, Rosenbaum JN, Nasrallah IM, Nasrallah MP. Histologic, immunohistochemical, and molecular features of pituicytomas and atypical pituicytomas. Acta Neuropathol Commun. 2019; 7:69. PMID: 31046843.
3. Lee EB, Tihan T, Scheithauer BW, Zhang PJ, Gonatas NK. Thyroid transcription factor 1 expression in sellar tumors: a histogenetic marker? J Neuropathol Exp Neurol. 2009; 68:482–488. PMID: 19525896.
4. Shim HK, Cha SH, Cho WH, Park SH. Pituicytoma with significant tumor vascularity mimicking pituitary macroadenoma. Brain Tumor Res Treat. 2017; 5:110–115. PMID: 29188213.
5. Kowalski RJ, Prayson RA, Mayberg MR. Pituicytoma. Ann Diagn Pathol. 2004; 8:290–294. PMID: 15494936.
6. Liss L, Kahn EA. Pituicytoma, tumor of the sella turcica; a clinicopathological study. J Neurosurg. 1958; 15:481–488. PMID: 13576191.
7. Antoni N. Gliomas of the neurohypophysis and hypophysial stalk; a preliminary report. J Neurosurg. 1950; 7:521–531. PMID: 14795256.
8. Scothorne CM. A glioma of the posterior lobe of the pituitary gland. J Pathol Bacteriol. 1955; 69:109–112. PMID: 13243176.
9. Salge-Arrieta FJ, Carrasco-Moro R, Rodríguez-Berrocal V, et al. Clinical features, diagnosis and therapy of pituicytoma: an update. J Endocrinol Invest. 2019; 42:371–384. PMID: 30030746.
10. Brat DJ, Scheithauer BW, Staugaitis SM, Holtzman RN, Morgello S, Burger PC. Pituicytoma: a distinctive low-grade glioma of the neurohypophysis. Am J Surg Pathol. 2000; 24:362–368. PMID: 10716149.
11. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820. PMID: 27157931.
12. Shibuya M. Welcoming the new WHO classification of pituitary tumors 2017: revolution in TTF-1-positive posterior pituitary tumors. Brain Tumor Pathol. 2018; 35:62–70. PMID: 29500747.
13. Zygourakis CC, Rolston JD, Lee HS, Partow C, Kunwar S, Aghi MK. Pituicytomas and spindle cell oncocytomas: modern case series from the University of California, San Francisco. Pituitary. 2015; 18:150–158. PMID: 24823438.
14. Wang J, Liu Z, Du J, et al. The clinicopathological features of pituicytoma and the differential diagnosis of sellar glioma. Neuropathology. 2016; 36:432–440. PMID: 26919073.
15. Feng M, Carmichael JD, Bonert V, Bannykh S, Mamelak AN. Surgical management of pituicytomas: case series and comprehensive literature review. Pituitary. 2014; 17:399–413. PMID: 24037647.
16. Wolfe SQ, Bruce J, Morcos JJ. Pituicytoma: case report. Neurosurgery. 2008; 63:E173–E174. PMID: 18728556.
17. Hammoud DA, Munter FM, Brat DJ, Pomper MG. Magnetic resonance imaging features of pituicytomas: analysis of 10 cases. J Comput Assist Tomogr. 2010; 34:757–761. PMID: 20861781.
18. Gibbs WN, Monuki ES, Linskey ME, Hasso AN. Pituicytoma: diagnostic features on selective carotid angiography and MR imaging. AJNR Am J Neuroradiol. 2006; 27:1639–1642. PMID: 16971602.
19. Benveniste RJ, Purohit D, Byun H. Pituicytoma presenting with spontaneous hemorrhage. Pituitary. 2006; 9:53–58. PMID: 16703409.
20. Cossu G, Dimitriou J, Brouland JP, Daniel RT, Messerer M. An exceptional presentation of pituicytoma apoplexy: a case report. Oncol Lett. 2018; 16:643–647. PMID: 29928451.
21. Kim YG, Park YS. Second-stage transsphenoidal approach (TSA) for highly vascular pituicytomas in children. Childs Nerv Syst. 2015; 31:985–989. PMID: 25771921.
Fig. 1
Radiologic features of pituicytoma. A-C: Sellar MRI T1-weighted image shows isointense cyst wall with some parenchyma in depending position (A, sagittal view) and slightly hyperintense cyst content (B, axial view), with moderate hyperintensity of cyst wall at contrast image (C, sagittal). D: Postoperative precontrast CT scan in sagittal view.
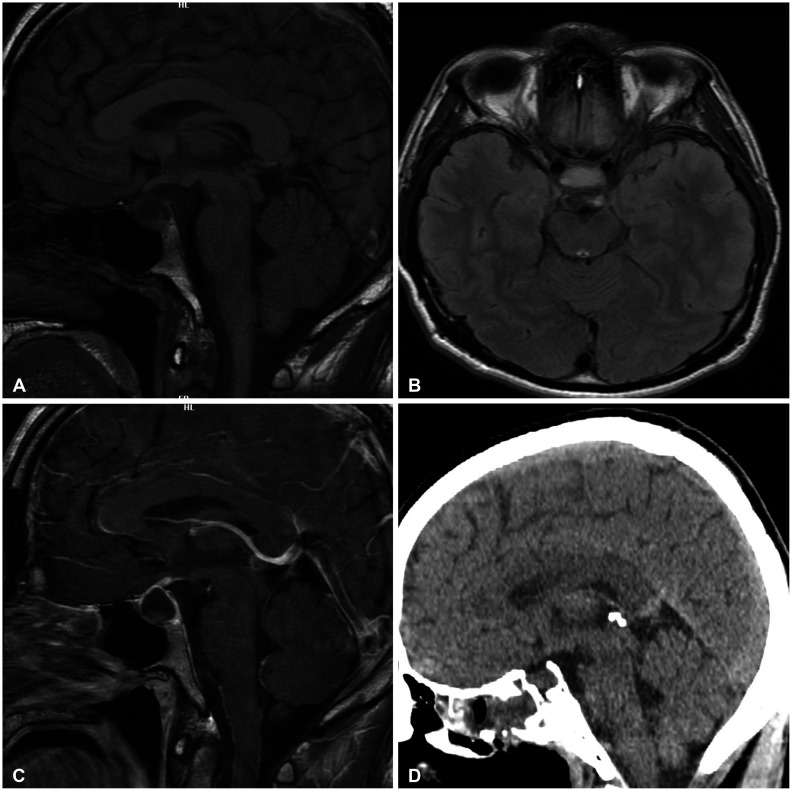
Fig. 2
Tissue morphology of pituicytoma. A: Tumor cells are arranged in sheets and fascicles. The tumor cells are spindled to epithelioid with abundant eosinophilic cytoplasm (hematoxylin and eosin, ×100). B-D: Immunohistochemistry of tumors. B: The cells shows diffuse, strong nuclear positivity for thyroid transcription factor 1 (×40). C: The tumor cells are S-100 positive (×40). D: Tumor cells shows positivity for glial fibrillary acidic protein (×40).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download