1. Scott RM, Dickersin R, Wolpert SM, Twitchell T. Myxochondrosarcoma of the fourth ventricle. Case report. J Neurosurg. 1976; 44:386–389. PMID:
1249620.
2. Smith TW, Davidson RI. Primary meningeal myxochondrosarcoma presenting as a cerebellar mass: case report. Neurosurgery. 1981; 8:577–581. PMID:
7266799.
3. Salcman M, Scholtz H, Kristt D, Numaguchi Y. Extraskeletal myxoid chondrosarcoma of the falx. Neurosurgery. 1992; 31:344–348. PMID:
1513440.
4. Sato K, Kubota T, Yoshida K, Murata H. Intracranial extraskeletal myxoid chondrosarcoma with special reference to lamellar inclusions in the rough endoplasmic reticulum. Acta Neuropathol. 1993; 86:525–528. PMID:
8310804.
5. Sala F, Talacchi A, Beltramello A, Iuzzolino P, Bricolo A. Intracranial myxoid chondrosarcoma with early intradural growth. J Neurosurg Sci. 1998; 42:159–163. PMID:
10192057.
6. Chaskis C, Michotte A, Goossens A, Stadnik T, Koerts G, D'Haens J. Primary intracerebral myxoid chondrosarcoma. Case illustration. J Neurosurg. 2002; 97:228. PMID:
12134922.
7. González-Lois C, Cuevas C, Abdullah O, Ricoy JR. Intracranial extraskeletal myxoid chondrosarcoma: case report and review of the literature. Acta Neurochir (Wien). 2002; 144:735–740. PMID:
12181708.
8. Im SH, Kim DG, Park IA, Chi JG. Primary intracranial myxoid chondrosarcoma: report of a case and review of the literature. J Korean Med Sci. 2003; 18:301–307. PMID:
12692436.
9. O'Brien J, Thornton J, Cawley D, et al. Extraskeletal myxoid chondrosarcoma of the cerebellopontine angle presenting during pregnancy. Br J Neurosurg. 2008; 22:429–432. PMID:
18568733.
10. Sorimachi T, Sasaki O, Nakazato S, Koike T, Shibuya H. Myxoid chondrosarcoma in the pineal region. J Neurosurg. 2008; 109:904–907. PMID:
18976082.
11. Dulou R, Chargari C, Dagain A, et al. Primary intracranial extraskeletal myxoid chondrosarcoma. Neurol Neurochir Pol. 2012; 46:76–81. PMID:
22426765.
12. Park JH, Kim MJ, Kim CJ, Kim JH. Intracranial extraskeletal myxoid chondrosarcoma: case report and literature review. J Korean Neurosurg Soc. 2012; 52:246–249. PMID:
23115670.
13. Qin Y, Zhang HB, Ke CS, et al. Primary extraskeletal myxoid chondrosarcoma in cerebellum: a case report with literature review. Medicine (Baltimore). 2017; 96:e8684. PMID:
29381948.
14. Velz J, Agaimy A, Frontzek K, et al. Molecular and clinicopathologic heterogeneity of intracranial tumors mimicking extraskeletal myxoid chondrosarcoma. J Neuropathol Exp Neurol. 2018; 77:727–735. PMID:
29924341.
15. Jroundi L, Kacemi L, Bounhir B, Chami I, Boujida N, Bacadi D. Intracerebral chondrosarcoma in a 12 year old child. J Radiol. 2005; 86:173–175. PMID:
15798629.
16. Korten AG, ter Berg HJ, Spincemaille GH, van der Laan RT, Van de Wel AM. Intracranial chondrosarcoma: review of the literature and report of 15 cases. J Neurol Neurosurg Psychiatry. 1998; 65:88–92. PMID:
9667567.
17. Sangoi AR, Dulai MS, Beck AH, Brat DJ, Vogel H. Distinguishing chordoid meningiomas from their histologic mimics: an immunohistochemical evaluation. Am J Surg Pathol. 2009; 33:669–681. PMID:
19194275.
18. Oliveira AM, Sebo TJ, McGrory JE, Gaffey TA, Rock MG, Nascimento AG. Extraskeletal myxoid chondrosarcoma: a clinicopathologic, immunohistochemical, and ploidy analysis of 23 cases. Mod Pathol. 2000; 13:900–908. PMID:
10955458.
19. Wernicke M, Piñeiro LC, Caramutti D, et al. Breast cancer stromal myxoid changes are associated with tumor invasion and metastasis: a central role for hyaluronan. Mod Pathol. 2003; 16:99–107. PMID:
12591961.
20. Harrison BT, Dillon DA. An update of mucinous lesions of the breast. Surg Pathol Clin. 2018; 11:61–90. PMID:
29413660.
21. Drilon AD, Popat S, Bhuchar G, et al. Extraskeletal myxoid chondrosarcoma: a retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer. 2008; 113:3364–3371. PMID:
18951519.
22. Stacchiotti S, Ferrari S, Redondo A, et al. Pazopanib for treatment of advanced extraskeletal myxoid chondrosarcoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019; 20:1252–1262. PMID:
31331701.

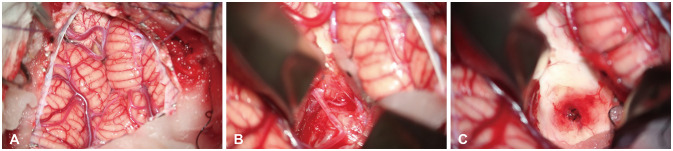
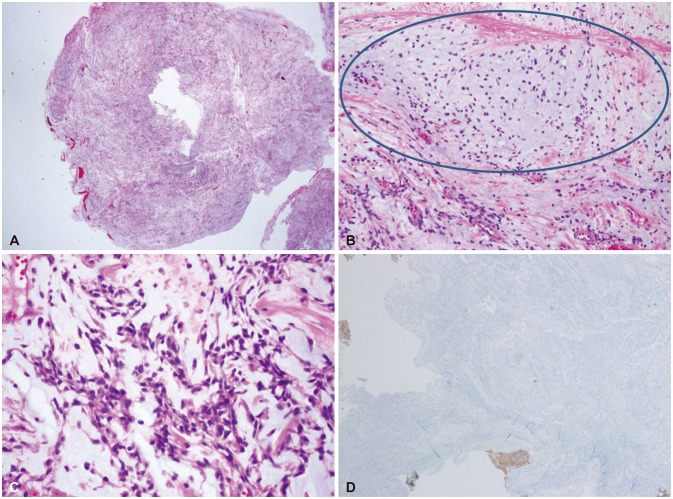
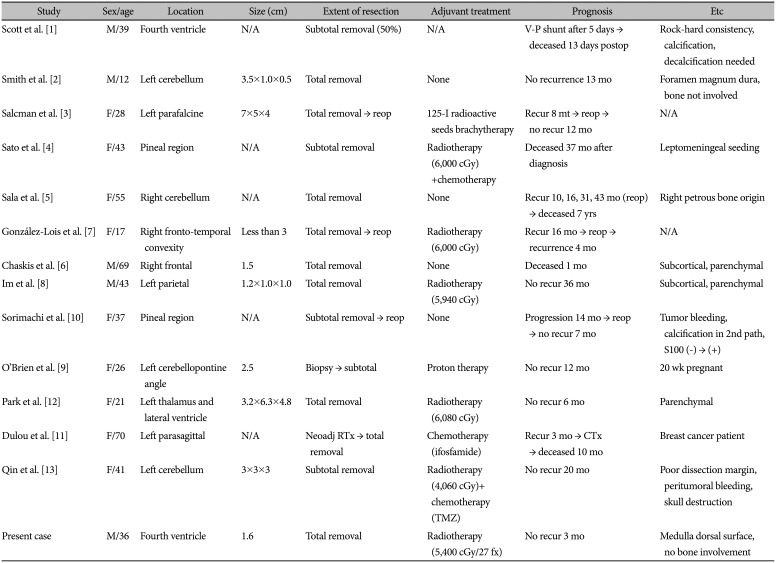
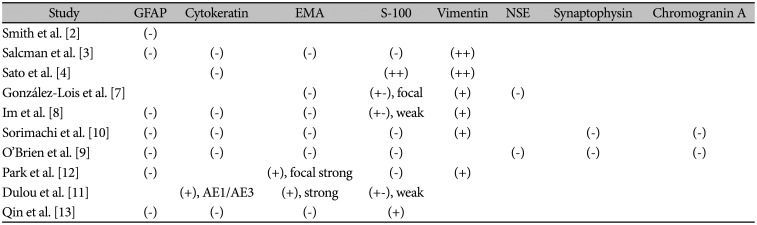




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download