Abstract
Pulmonary blastoma is a rare type of primary lung cancer that accounts for only 0.25%–0.5% of all lung malignancies. Pulmonary blastoma consists of three subgroups: classic biphasic pulmonary blastoma (CBPB), pleuropulmonary blastoma, and well-differentiated fetal adenocarcinoma. Due to the rarity of the tumor, metastatic brain tumor from CBPB is extremely rare, and only 13 cases, including our case, have been reported. A 60-year-old woman who underwent left upper lobectomy of the lung because of pathologically diagnosed as CBPB 5 months ago, suddenly lost consciousness and presented with stupor mental status. The emergent CT scan showed a large, 51 mL, intracerebral hemorrhage on left parieto-occipital lobe with midline shifting. The patient underwent emergent craniotomy, and a hypervascular tumor was identified during the operation. Histopathologic examination reported metastatic pulmonary blastoma, CBPB. The patient has been in a vegetative state, but there has been no evidence of recurrence over a 6-month follow-up period. We report a rare case of brain metastasis from CBPB presenting with altered mentality due to massive tumor bleeding. This is the only reported case of brain metastasis from CBPB presenting with acute intracerebral hemorrhage.
Pulmonary blastoma is a rare type of malignant lung neoplasm that accounts for only 0.25%–0.5% of all lung malignancies [1]. Pulmonary blastoma consists of three subgroups: classic biphasic pulmonary blastoma (CBPB), pleuropulmonary blastoma (PPB), and well-differentiated fetal adenocarcinoma (WDFA) [1]. Among these, CBPB is classified under sarcomatoid carcinoma of the lung along with pleomorphic carcinoma and carcinosarcoma according to the 2015 WHO Classification of Lung Tumors [2]. Unlike PPB and WDFA, CBPB is a biphasic tumor consisting of fetal adenocarcinoma (typically low grade) and primitive mesenchymal stroma [2].
CBPB shows poor prognosis, and the 5-year survival rate is only 16% [3]. CBPB recurs with a rate of 43% and is inclined to metastasize to the mediastinum, pleura, diaphragm, liver, heart, soft tissues of the extremities, and even to the brain [1].
Due to the rarity of CBPB, metastatic brain tumor from CBPB is extremely rare. Brain metastasis from pulmonary blastoma was first reported by Barson et al. [4] in 1968. Before 2015, only 11 cases of metastatic brain tumor from pulmonary blastoma were reported [5]. Based on the literature, to date, a total of 13 cases have been reported, including our case [456789101112131415].
We report a rare case of a 60-year-old female patient who presented with intracerebral hemorrhage (ICH) from CBPB brain metastasis.
A 60-year-old woman underwent left upper lobectomy of the lung due to pathologically diagnosed CBPB 5 months ago. At that time, brain MRI was obtained for CBPB staging work-up; however, there was no abnormality (Fig. 1A). After lung surgery, the patient fully recovered and patent foramen ovale (PFO) was diagnosed through echocardiography; thus, PFO closure was planned.
Upon intervention, the patient suddenly lost consciousness and presented with stuporous mental status. An emergent CT scan of the brain showed a large amount (51 mL) of ICH on left parieto-occipital lobe with a 1.5 cm-sized internal low-density lesion which implicated lesional hemorrhage due to tumorous condition (Fig. 1B). The ICH showed a severe mass effect; thus, emergent craniotomy was performed.
The patient underwent left parieto-occipital craniotomy and hematoma evacuation with tumor removal. Upon dura opening, severe brain swelling was noted. A large amount of hematoma was evacuated, and a grayish hypervascular tumor, which showed a hard consistency, was identified inside the hematoma (Fig. 2). Therefore, gross total resection of the tumor and hematoma evacuation were performed (Fig. 3A). However, an intraoperative CT scan showed ICH in the left temporal lobe (Fig. 3B). Subsequently, an immediate left decompressive hemicraniectomy and removal of the temporal lobe ICH were performed (Fig. 3C and D).
Histopathologic examination reported metastatic pulmonary blastoma, CBPB. In hematoxylin and eosin staining, the tumor showed both glandular epithelial components and blastomal stromal component. In an immunohistochemical study, the tumor showed positivity for nuclear beta-catenin and thyroid transcription factor-1 negativity for synaptophysin (Fig. 4).
Two months postoperatively, the patient was transferred to a rehabilitation hospital for conservative care. Until discharge, she has been in a vegetative state; thus, further adjuvant treatment has been put on hold considering her general condition. There has been no evidence of recurrence over a 6-month follow-up period.
Pulmonary blastoma is an uncommon type of primary malignant lung cancer with high malignancy, which represents only 0.25%–0.5% of all lung malignancies [1]. Despite its rarity, over 200 cases of pulmonary blastoma have been reported since 1945 [16]. However, cerebral metastasis from pulmonary blastoma is extremely rare. Since the first description of cerebral metastasis from pulmonary blastoma in 1968 by Barson et al. [4], metastatic brain tumor has been reported in only 13 cases of pulmonary blastoma, including this case (Table 1) [456789101112131415]. Owing to its rarity, there are no studies on metastatic brain tumor from pulmonary blastoma regarding the tendency of multiple or solitary metastasis, propensity of bleeding, and treatment strategy.
Among the 13 cases of cerebral metastasis, only two cases, including this case, had hemorrhagic metastasis [9]. The most common clinical presentation of metastatic brain tumor from pulmonary blastoma was headache. Only three of the 13 patients developed motor weakness due to metastasis in eloquent areas [4911]. However, this report presents the first case of altered mentality due to metastatic tumor bleeding with severe cerebral swelling.
Due to the rarity of pulmonary blastoma, there is no established treatment strategy. Furthermore, with only a few case reports of cerebral metastasis, there is no established treatment strategy for cerebral metastasis either. Based on previous reports regarding the treatment of brain metastasis from pulmonary blastoma, there was a partial response to radiotherapy [6911]. Furthermore, response to stereotactic radiosurgery combined with whole-brain radiotherapy was reported by Schwitter et al. [12]. According to these reports, adjuvant radiotherapy can be efficient in treating metastatic brain tumor from pulmonary blastoma.
In our case, since the patient collapsed with altered mentality due to tumor bleeding in the cerebral hemisphere, there was no choice but emergent craniotomy, tumor removal, and hematoma evacuation. Radiotherapy and chemotherapy were halted due to an unrecovered mentality based on risk benefit. Instead, the patient has been observed closely without adjuvant radiotherapy or chemotherapy, and there has been no evidence of recurrence over 6 months.
Due to the rarity of pulmonary blastoma and cerebral metastasis of pulmonary blastoma, a treatment strategy has not been established, and the prognosis of metastatic brain tumor from pulmonary blastoma is unclear. Herein, we report our case of brain metastasis from a pulmonary blastoma, hoping this data could be a valuable clinical asset. More clinical data should be assembled to establish a treatment strategy for pulmonary blastoma with brain metastasis.
In conclusion, pulmonary blastoma is a rare type of primary malignant lung cancer, and brain metastasis of pulmonary blastoma is extremely rare. Only 13 cases, including this case, have been reported to date. There are various presentations of brain metastasis; however, this case was the first case of sudden collapse due to hemorrhage in a metastatic brain tumor from pulmonary blastoma. Despite the rarity of the case of metastatic brain tumor from pulmonary blastoma, further studies regarding treatment of pulmonary blastoma and its metastasis to the brain should be conducted owing to the constant occurrence of the tumor. We hope our case could be a valuable clinical asset.
Notes
Author Contributions:
Conceptualization: Joonho Byun, Sanghyeok Park.
Data curation: Sanghyeok Park.
Formal analysis: Joonho Byun, Sanghyeok Park.
Investigation: Sanghyeok Park.
Methodology: Sang Woo Song, Young-Hoon Kim, Jeong Hoon Kim.
Project administration: Sanghyeok Park.
Supervision: Chang-Ki Hong, Jeong Hoon Kim.
Validation: Sanghyeok Park, Sang Woo Song, Young-Hoon Kim.
Visualization: Sanghyeok Park, Joonho Byun.
Writing—original draft: Sanghyeok Park, Joonho Byun.
Writing—review & editing: all authors.
Ethics Statement
The institutional review board (IRB) exempted informed consent due to only descriptive nature of the case report and no risk for harm to the patient, and this report was conducted according to the guidelines of the Declaration of Helsinki for biomedical research.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Van Loo S, Boeykens E, Stappaerts I, Rutsaert R. Classic biphasic pulmonary blastoma: a case report and review of the literature. Lung Cancer. 2011; 73:127–132. PMID: 21513998.
2. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015; 10:1243–1260. PMID: 26291008.
3. Koss MN, Hochholzer L, O'Leary T. Pulmonary blastomas. Cancer. 1991; 67:2368–2381. PMID: 1849449.
4. Barson AJ, Jones AW, Lodge KV. Pulmonary blastoma. J Clin Pathol. 1968; 21:480–485. PMID: 5697348.
5. Xiu Y, Jiang L, Liu W. Classic biphasic pulmonary blastoma with brain and axillary metastases: a case report with molecular analysis and review of literature. Int J Clin Exp Pathol. 2015; 8:983–988. PMID: 25755806.
6. Bini A, Ansaloni L, Grani G, et al. Pulmonary blastoma: report of two cases. Surg Today. 2001; 31:438–442. PMID: 11381509.
7. Teixeira A, Vieira C, Sousa N, et al. Biphasic pulmonary blastoma with germ cell differentiation: a challenge in diagnosis and treatment. Acta Med Port. 2011; 24 Suppl 3:685–688. PMID: 22856413.
8. Kilic D, Yilmaz C, Tepeoglu M, Vural C, Caner H. Biphasic pulmonary blastoma associated with cerebral metastasis. Turk Neurosurg. 2016; 26:169–172. PMID: 26768884.
9. Kouvaris JR, Gogou PV, Papacharalampous XN, Kostara HJ, Balafouta MJ, Vlahos LJ. Solitary brain metastasis from classic biphasic pulmonary blastoma: a case report and review of the literature. Onkologie. 2006; 29:568–570. PMID: 17202827.
10. Takahashi K, Kohno T, Matsumoto S, et al. Clonality and heterogeneity of pulmonary blastoma from the viewpoint of genetic alterations: a case report. Lung Cancer. 2007; 57:103–108. PMID: 17350138.
11. Kawasaki K, Yamamoto K, Suzuki Y, Saito H. Surgery and radiation therapy for brain metastases from classic biphasic pulmonary blastoma. BMJ Case Rep. 2014; 2014:bcr2014203990.
12. Schwitter M, Potocnik P, von Moos R, Frick H, Furrer M, Cathomas R. Dyspnoea and a lung mass in a young female 2 weeks after Caesarean delivery. Eur Respir J. 2011; 38:465–467. PMID: 21804162.
13. Shimotakahara T, Hirata S, Matsumoto H, et al. [A case of pulmonary blastoma]. Kyobu Geka. 1992; 45:339–342. PMID: 1564812.
14. Dopfer R, Leidig E, Treuner J, Reifferscheid P, Fischbach H, Niethammer D. Pulmonary blastoma in a 4 year old girl. Z Kinderchir. 1981; 33:182–186. PMID: 7282085.
15. Kliem V, Bügge M, Leimenstoll K, Maschek H. Pulmonary blastoma--a rare tumour. Clin Investig. 1992; 70:927–931.
16. Adluri RK, Boddu SR, Martin-Ucar A, Duffy JP, Beggs FD, Morgan WE. Pulmonary blastoma--a rare tumor with variable presentation. Eur J Cardiothorac Surg. 2006; 29:236–239. PMID: 16387506.
Fig. 1
Radiologic findings of metastatic pulmonary blastoma. A: Brain magnetic resonance image with T1-gadolinium enhancement taken 5 months ago for initial pulmonary blastoma staging. There is no evidence of brain metastasis. B: Brain CT scan at initial diagnosis: hyperacute stage of intracerebral hemorrhage in the left parieto-occipital lobe with 1.4 cm midline shifting. There is a 1.5 cm-sized low-density lesion within the hematoma, indicating tumor bleeding (red arrow). Loss of gyral marking on the left hemisphere also indicates severe brain swelling.
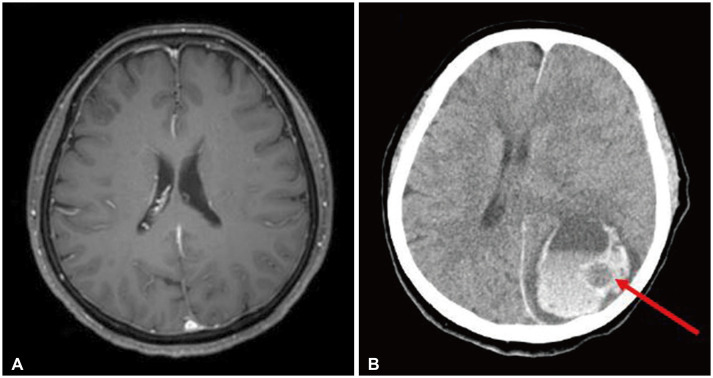
Fig. 2
Intraoperative findings of metastatic pulmonary blastoma. A: Intraoperative finding of intracerebral hemorrhage. B and C: After hematoma evacuation, the grayish and hypervascular tumor is identified. Both yellow arrows indicate the tumor. D: Final photo after gross total removal of the tumor and hematoma evacuation.
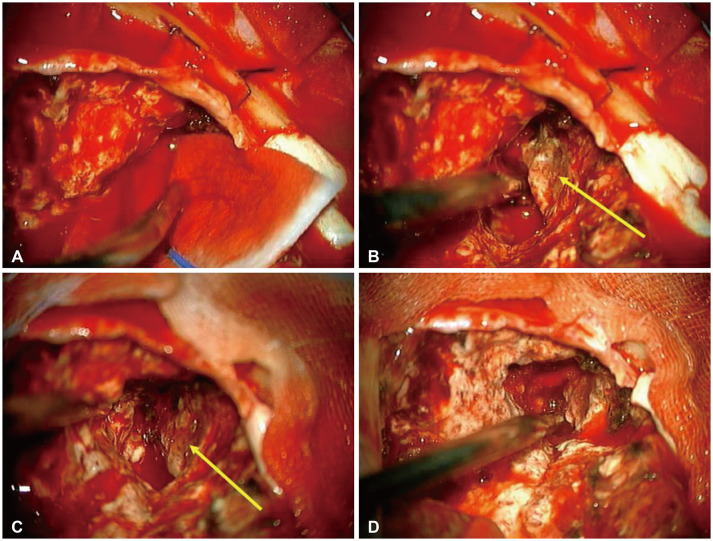
Fig. 3
Post-procedural intraoperative and postoperative CT scan. A: Hematoma and tumor on the left parieto-occipital lobe was removed. B: Newly developed large amount left temporal lobe hematoma was noted. C and D: Postoperative CT scan of left decompressive hemicraniectomy and temporal lobe hematoma removal. Hematoma was totally removed.
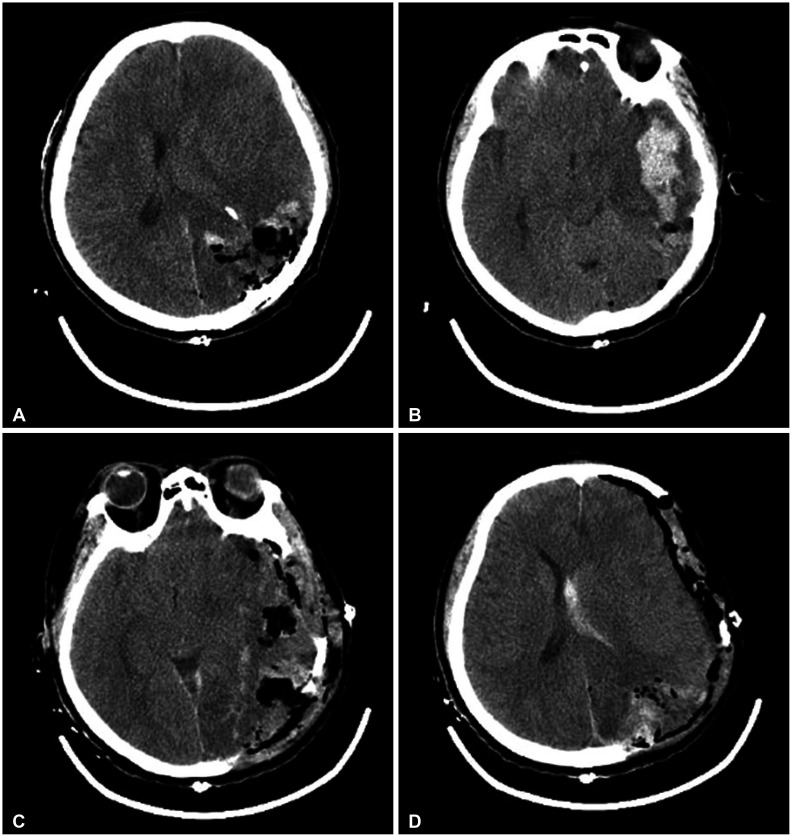
Fig. 4
Pathologic findings of brain metastasis from pulmonary blastoma. A: Hematoxylin and eosin stain of metastatic brain tumor from pulmonary blastoma (×400). The tumor shows both glandular epithelial components and blastemal stromal component (circle). B: Immunohistochemically, the tumor shows focal nuclear positive for glandular epithelial component and diffuse nuclear positive for the blastemal stromal component (circle) of beta-catenin (×400). C: The tumor is focal immunopositive for thyroid transcription factor-1 (×400). D: The tumor is completely immunonegative for synaptophysin (×400).
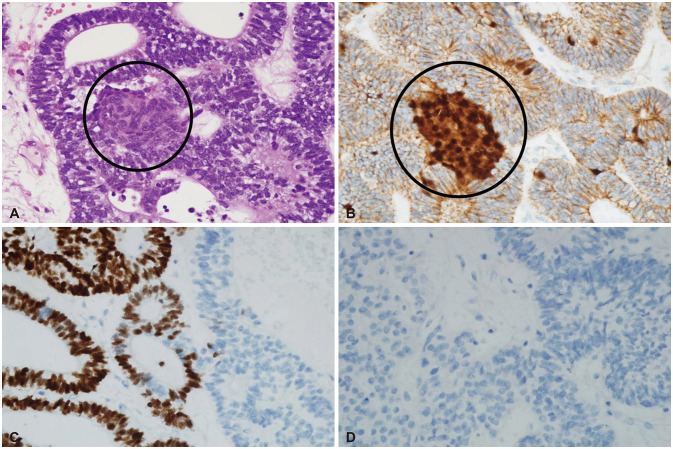
Table 1
All cases of classic biphasic pulmonary blastoma with brain metastasis presented in the literature
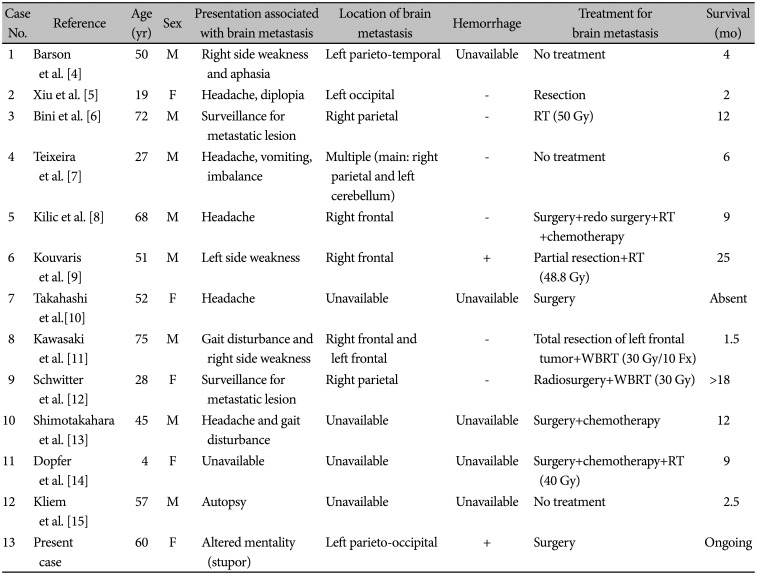
| Case No. | Reference | Age (yr) | Sex | Presentation associated with brain metastasis | Location of brain metastasis | Hemorrhage | Treatment for brain metastasis | Survival (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | Barson et al. [4] | 50 | M | Right side weakness and aphasia | Left parieto-temporal | Unavailable | No treatment | 4 |
| 2 | Xiu et al. [5] | 19 | F | Headache, diplopia | Left occipital | - | Resection | 2 |
| 3 | Bini et al. [6] | 72 | M | Surveillance for metastatic lesion | Right parietal | - | RT (50 Gy) | 12 |
| 4 | Teixeira et al. [7] | 27 | M | Headache, vomiting, imbalance | Multiple (main: right parietal and left cerebellum) | - | No treatment | 6 |
| 5 | Kilic et al. [8] | 68 | M | Headache | Right frontal | - | Surgery+redo surgery+RT+chemotherapy | 9 |
| 6 | Kouvaris et al. [9] | 51 | M | Left side weakness | Right frontal | + | Partial resection+RT (48.8 Gy) | 25 |
| 7 | Takahashi et al. [10] | 52 | F | Headache | Unavailable | Unavailable | Surgery | Absent |
| 8 | Kawasaki et al. [11] | 75 | M | Gait disturbance and right side weakness | Right frontal and left frontal | - | Total resection of left frontal tumor+WBRT (30 Gy/10 Fx) | 1.5 |
| 9 | Schwitter et al. [12] | 28 | F | Surveillance for metastatic lesion | Right parietal | - | Radiosurgery+WBRT (30 Gy) | >18 |
| 10 | Shimotakahara et al. [13] | 45 | M | Headache and gait disturbance | Unavailable | Unavailable | Surgery+chemotherapy | 12 |
| 11 | Dopfer et al. [14] | 4 | F | Unavailable | Unavailable | Unavailable | Surgery+chemotherapy+RT (40 Gy) | 9 |
| 12 | Kliem et al. [15] | 57 | M | Autopsy | Unavailable | Unavailable | No treatment | 2.5 |
| 13 | Present case | 60 | F | Altered mentality (stupor) | Left parieto-occipital | + | Surgery | Ongoing |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download