Abstract
A 25-year-old female presented with a generalized tonic-clonic seizure. She had no previous history of seizures. A brain magnetic resonance imaging scan revealed a solitary enhancing mass in the right fronto-parietal cortex. During surgery, the mass was noted to be pure cortical with no connection to the ventricular lining. The tumor was completely resected. After surgery, the patient had no further seizures. The biopsy result showed a supratentorial ependymoma, which was C11orf95-RELA-fusion-positive.
Ependymomas (EPNs) are a neuroepithelial malignancy that constitute about 2%–9% of all neuroepithelial tumors. They are most frequently seen in children and young adults [12]. Only a third of them are supratentorial [3]. The most common subtype of supratentorial ependymoma (STE) is the C11orf95-RELA-fusion type [4]. Half of STE are extraventricular; however, they usually connect to the ventricular lining [5]. EPNs with no connection to the ventricular lining are extremely rare [56]. We report a case of supratentorial cortical ependymoma (CE) with C11orf95-RELA-fusion positive in a young female and literature review.
A 25-year-old female presented to our department after a generalized tonic-clonic seizure. She had no prior history of seizures. The brain MRI scans showed a strongly enhancing cortical mass about 3×2×2 cm in size with peri-lesional edema and with no definite dural tail sign (Fig. 1). The initial radiological diagnoses were meningioma or hemangiopericytoma. Although it was a small tumor, since it was causing seizures, we decided to perform total surgical resection as the primary treatment.
A right fronto-parietal craniotomy was performed, and we achieved gross total removal of the tumor. Contrary to our expectation, there was no adhesion between the dura-mater and the tumor. The tumor was well-demarcated, marginated from right fronto-parietal cortex, and encapsulated. It was highly vascularized, and friable solid mass. The tumor had minimal adhesions attaching to the arachnoid membrane, but no invasion of the brain parenchyma was seen (Fig. 2). The tumor was friable; therefore, it was thoroughly aspirated with a cavitron ultrasonic surgical aspirator. Gross total removal without any injury of the peri-lesional area was achieved. After surgery, the patient had no further seizures. Postoperative MRI scans showed no evidence of a residual mass.
In histological study, we found that the tumor had a clear cell feature which requires some differentiation such as clear cell EPN, clear cell meningioma or oligodendroglioma. Hematoxylin and eosin (H&E) staining showed a sheet of uniform cells with round nuclei, finely dispersed chromatin, clear cytoplasm, discrete cytoplasmic borders, rare mitosis, no necrosis (Fig. 3). Immunohistochemically, the epithelial membrane antigen (EMA) was positive with a dot-like pattern (Fig. 4A), GFAP and Olig2 were focal positive (Fig. 4B and C), and L1CAM was strongly positive (Fig. 4D). Analysis of 1p/19q codeletion fluorescence in situ hybridization (FISH) showed no deletion with polysomy. Ki-67 labeling index was 14.3%, S-100 was positive, vimentin was positive, and IDH1 gene mutation was not detected. Histological and immunohistochemical study allowed the tumor to be diagnosed as EPN with C11orf95-RELA-fusion.
MRI scans that were performed at the time of 10 months after tumor removal operation did not show any significant recurrence and the peri-lesional edema that was seen in preoperative MRI scans was no longer visible. And the patient maintained the seizure free state.
EPNs are relatively rare glial tumors that can occur anywhere in the central nervous system [17]. According to World Health Organization (WHO), EPNs are classified into subgroups by their molecular features [8]. Subependymoma and myxopapillary EPNs are classified as WHO grade I. Papillary EPN, clear cell EPN, and tanycytic EPN are classified as WHO grade II, and anaplastic EPN is classified as WHO grade III [910]. In addition, C11orf95-RELA-fusion positive EPN was newly classified as WHO grade II/III in 2016 [910].
There are two major subtypes of STE classified by their molecular features: C11orf95-RELA-fusion type and YAP-1-fusion type [9]. C11orf95-RELA-fusion EPN is the most common type, occurring in about 70% of cases [11]. However, it is not found in infratentorial or spinal EPNs [12]. The RELA gene encodes the NF-κB protein complex, which controls cell proliferation and apoptosis by its signal pathway [12]. Activation of the NF-κB signal pathway can contribute to tumor development by four mechanisms that suggested by Xia et al. [13]. It stimulates cell proliferation and prevents cell apoptosis, regulates angiogenesis, promotes tumor metastasis, and remodels tumor metabolism. The fusion of the C11orf95 gene—whose oncogenic function has not been clearly revealed—and the RELA can drive activation of the oncogenic NF-κB signaling in EPNs [11]. For these reasons, C11orf95-RELA-fusion STEs are thought to be having anaplastic features. And it can be considered that C11orf95-RELA-fusion STEs have a poorer prognosis than other types of EPN.
In several literatures, CEs are defined as EPNs that are located in the cerebral cortex and have no connection to the ventricular lining and that account for only about 3.5% to 5.7% of all EPNs [231415]. Especially, in some literatures like this case, it is called pure CEs to emphasize that they are limited only to the cerebral cortex [516].
Currently, CEs have been reported about 100 cases. In 2020, Wang et al. [14] reported a cohort study on 106 cases that have been reported till that date and 30 cases of CEs of their institute. In their study, seizure is the most common symptom, the frontal lobe or involving the frontal lobe are the most common site of CEs and relatively higher rate of C11orf95-RELA-fusion than that of other STEs that fusion rate is known as about 70%. They performed C11orf95-RELA-fusion test in 10 of their 30 cases that the test was available, and 9 cases were confirmed to be fusion positive by FISH [14]. The prognosis of CEs was favorable, whereas a poorer prognosis was expected than that of other STEs because CEs have higher rate of C11orf95-RELA-fusion and they have anaplastic features [14].
In 2019, Matsumoto et al. [15] reported their 8 cases and 84 cases previously reported CE cases. Their results were similar to those of Wang et al. [14]. They performed Sanger sequencing of cDNA for finding C11orf95-RELA-fusion in 5 of their 8 cases that the test was available, and all 5 cases were fusion positive. They also performed L1CAM stain, and 6 of 8 cases were positive [15].
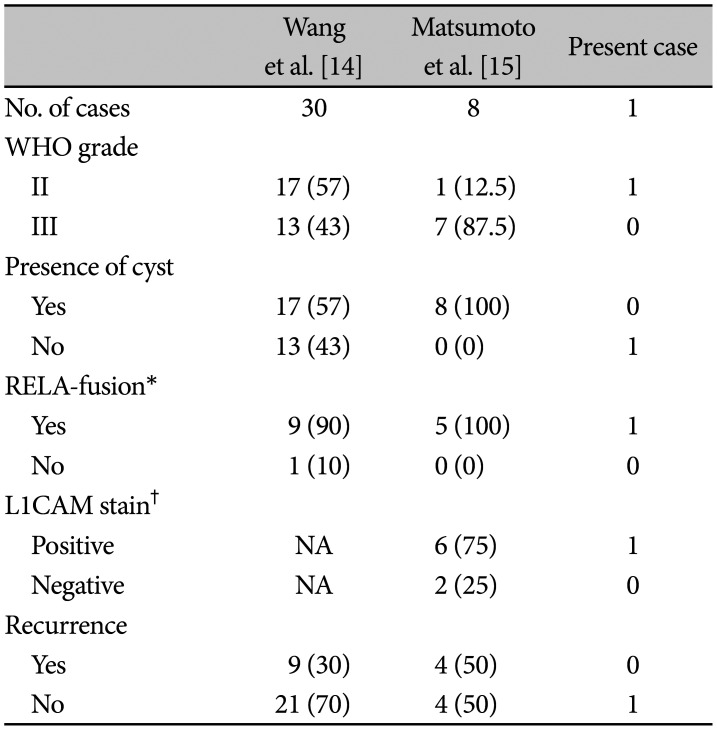
In those studies of two groups showed higher rate of C11orf95-RELA-fusion than other STEs but prognosis was favorable. They performed gross total resection in most of their cases, and it would have made it possible to overcome the expected poor prognosis (Table 1). And according to Matsumoto group’s result, although not all RELA-fusion positive were L1CAM stain positive, all L1CAM stain positive were confirmed to be RELA-fusion positive. This result suggests that the L1CAM staining can also be a powerful tool for screening C11orf95-RELA-fusion. While the tumor texture of the present case was pure solid, cystic lesions occupied the majority in both groups (25/38). There were 4 cases of dead outcome in Wang group, all of which had recurrences. This suggests that recurrence is an important factor in prognosis. The total recurrence rate in both groups was about 34% (13/38), but there was no recurrence in this case, which is thought to be because the GTR was easily achieved in this case as it was a pure cortical lesion with no definite parenchymal invasion (Table 2).
In the radiologic findings of this case, pure cortical type, absence of a cystic lesion, and with no connection to ventricle were factors that contributed to the difficulty in recognizing the tumor as an EPN. Because a pure cortical, extra-ventricular nature, and with no ventricular lining are rare findings in EPNs [517].
During surgery, a well-demarcated and friable mass was fully aspirated. There was no gross residual tumor and no definite invasion of the brain parenchyma. H&E staining showed that the tumor had clear cell features that suggested the possibility of EPN, clear cell meningioma, or oligodendroglioma. It was difficult to differentiate between EPN and clear cell meningioma because of the tumor had clear cell features and rare mitosis. However, it was possible to rule out clear cell meningioma through the findings that GFAP staining was positive, there was no dural adhesion during surgery and dural tailing was not prominent in MRI scans. Absence of 1p/19q codeletion in FISH analysis and IDH1 mutation allowed to easily exclude oligodendroglioma. The GFAP, EMA, S100, and vimentin staining showed all positive results that are characteristic immunochemical staining of EPNs. In addition, L1CAM staining showed a strong positive result that strongly suggested the tumor was C11orf95-RELA-fusion positive [1118].
The standard treatment for EPNs is total surgical resection. As mentioned earlier, total surgical resection is very important because C11orf95-RELA-fusion EPN has anaplastic features. Radiation therapy (RT) can be considered for remnants or prevention of recurrence. The role of chemotherapy is limited [8]. There was no evidence of residual tumor mass on the postoperative MRI scans and no evidence of recurrence on the follow-up MRI scans of the patient in this case; however, RT or re-operation would be considered if there is evidence of recurrence or residual tumor at further follow-up. In particular, since C11orf95-RELA-fusion EPN has anaplastic features that can spontaneously activate NF-κB signaling, careful follow-up is required [19].
EPN, as in this case, is difficult to diagnose due to its extremely rare features. Therefore, diagnosis and surgical resection should be performed at the same time. Furthermore, CEs may have higher rate of C11orf95-RELA-fusion that has anaplastic features, but CEs have favorable prognosis through gross total resection of tumor and adjuvant radiotherapy that may have made it possible to overcome the expected poor prognosis. This is why surgical total resection is the most important procedure as the first treatment. And, in terms of epidemiologic aspects of EPNs, close follow-up is required because it mainly occur in children and young adults.
Notes
Author Contributions:
Conceptualization: Sang-Woo Lee.
Data curation: Sang-Woo Lee.
Formal analysis: Sang-Woo Lee.
Investigation: Sang-Woo Lee.
Methodology: Min-Seok Lee, Sang-Jun Suh.
Project administration: Jeong-Ho Lee.
Resources: Jeong-Ho Lee.
Supervision: Jeong-Ho Lee.
Validation: Yoon-Soo Lee.
Visualization: Jin-Wook Kim.
Writing—original draft: Sang-Woo Lee.
Writing—review & editing: Sang-Woo Lee.
Ethics Statement
The Institutional Review Board of Daegu Fatima Hospital exempted the requirement for informed consent due to the retrospective nature of the study and the minimal risk for the patient (DFE21ORIO097). This report was conducted according to the guidelines of the Declaration of Helsinki for biomedical research.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Sun S, Wang J, Zhu M, et al. Clinical, radiological, and histological features and treatment outcomes of supratentorial extraventricular ependymoma: 14 cases from a single center. J Neurosurg. 2018; 128:1396–1402. PMID: 28686116.
2. Van Gompel JJ, Koeller KK, Meyer FB, et al. Cortical ependymoma: an unusual epileptogenic lesion. J Neurosurg. 2011; 114:1187–1194. PMID: 21235315.
3. Sallam YT, Zhang Q, Pandey SK. Cortically based cystic supratentorial RELA fusion-positive ependymoma: a case report with unusual presentation and appearance and review of literature. Radiol Case Rep. 2020; 15:2495–2499. PMID: 33033550.
4. Zhu JJ, Jillette N, Li XN, Cheng AW, Lau CC. C11orf95-RELA reprograms 3D epigenome in supratentorial ependymoma. Acta Neuropathol. 2020; 140:951–960. PMID: 32909151.
5. Yadav YR, Neha , Chandrakar SK. Pure cortical supratentorial extraventricular ependymoma. Neurol India. 2009; 57:213–215. PMID: 19439861.
6. Wu X, Liang P, Zhai X. Supratentorial atypical ectopic ependymoma in a child: a case report. Int J Clin Exp Med. 2017; 10:14827–14833.
7. Elsharkawy AE, Abuamona R, Bergmann M, Salem S, Gafumbegete E, Röttger E. Cortical anaplastic ependymoma with significant desmoplasia: a case report and literature review. Case Rep Oncol Med. 2013; 2013:354873. PMID: 24455359.
8. Jung TY, Jung S, Kook H, Baek HJ. Treatment decisions of World Health Organization grade II and III ependymomas in molecular era. J Korean Neurosurg Soc. 2018; 61:312–318. PMID: 29742878.
9. Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015; 27:728–743. PMID: 25965575.
10. Gupta A, Dwivedi T. A simplified overview of World Health Organization classification update of central nervous system tumors 2016. J Neurosci Rural Pract. 2017; 8:629–641. PMID: 29204027.
11. Parker M, Mohankumar KM, Punchihewa C, et al. C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature. 2014; 506:451–455. PMID: 24553141.
12. Pietsch T, Wohlers I, Goschzik T, et al. Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-κB signaling pathway. Acta Neuropathol. 2014; 127:609–611. PMID: 24562983.
13. Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014; 2:823–830. PMID: 25187272.
14. Wang Q, Cheng J, Li J, et al. The survival and prognostic factors of supratentorial cortical ependymomas: a retrospective cohort study and literature-based analysis. Front Oncol. 2020; 10:1585. PMID: 32974195.
15. Matsumoto Y, Ichikawa T, Kurozumi K, Otani Y, Date I. Clinicopathological and genetic features of supratentorial cortical ependymomas. World Neurosurg. 2019; 129:e417–e428. PMID: 31150846.
16. Bijwe S, Ansari S, Jadhav V, Palande D. Pure cortical ependymoma: a rare entity. Asian J Neurosurg. 2015; 10:162–165. PMID: 25972957.
17. Berhili S, Aissa A, Kadiri S, et al. Extra-axial ependymoma of the cerebral convexity: a very rare intracranial adult tumor. Neuroradiol J. 2017; 30:281–285. PMID: 28059629.
18. Onishi S, Yamasaki F, Nakano Y, et al. RELA fusion-positive anaplastic ependymoma: molecular characterization and advanced MR imaging. Brain Tumor Pathol. 2018; 35:41–45. PMID: 29063976.
19. Nakamura T, Fukuoka K, Ikeda J, et al. Encouraging option of multistaged gross total resection for a C11orf-RelA fusion-positive supratentorial anaplastic ependymoma. Brain Tumor Pathol. 2017; 34:160–164. PMID: 28831588.
Fig. 1
Preoperative radiologic finding. A and B: Gadolinium-enhanced T1-weighted brain MRI scans show well enhancing pure cortical mass (white arrows) in size of 3×2×2 cm. C: T2-flair scan shows mass (white arrow) and peri-lesional edema (white arrowhead).
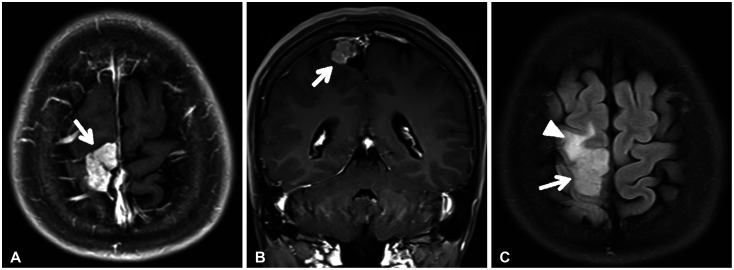
Fig. 2
Intraoperative microscopic finding. A: Intraoperative photograph after dura-mater was opened shows tumor mass (asterisk). The mass was friable solid and highly vascularized, and had no definite adhesion between dura-mater. B: Intraoperative photograph after mass was totally removed shows no definite remnant and parenchymal invasion.
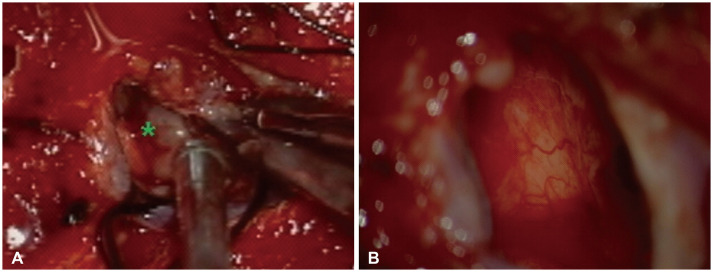
Fig. 3
Histopathological examination with hematoxylin and eosin staining shows a sheet of uniform cells with round nuclei and highly vascularized features (×40) (A) and shows nuclei are with finely dispersed chromatin, clear cytoplasm with well-defined cell membrane, discrete cytoplasmic borders, rare mitosis, no definite necrosis (B, ×100; C, ×200). The black arrows indicate clear cells (C).

Fig. 4
Immunohistochemical examination shows positive with a dot like pattern in EMA staining (×200) (A), focal positive in GFAP (×200) and Olig2 (×200) staining (B and C), and strongly positive in L1CAM staining (×200) (D).
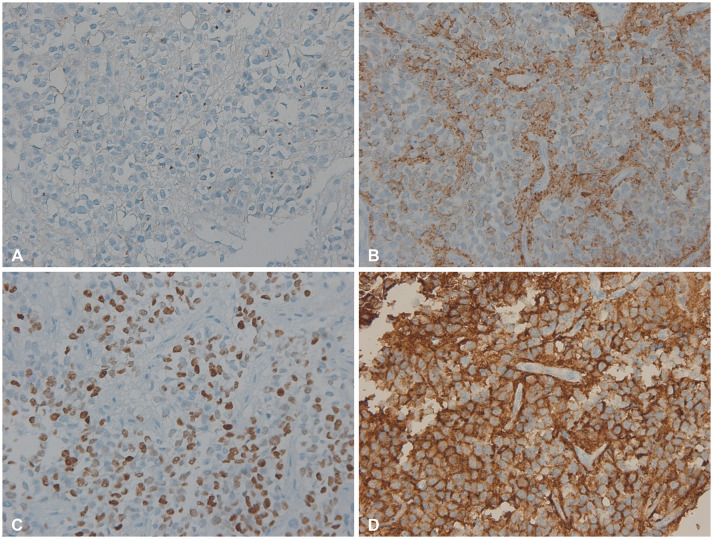
Table 1
A summary of cortical ependymoma cases from other groups and present case
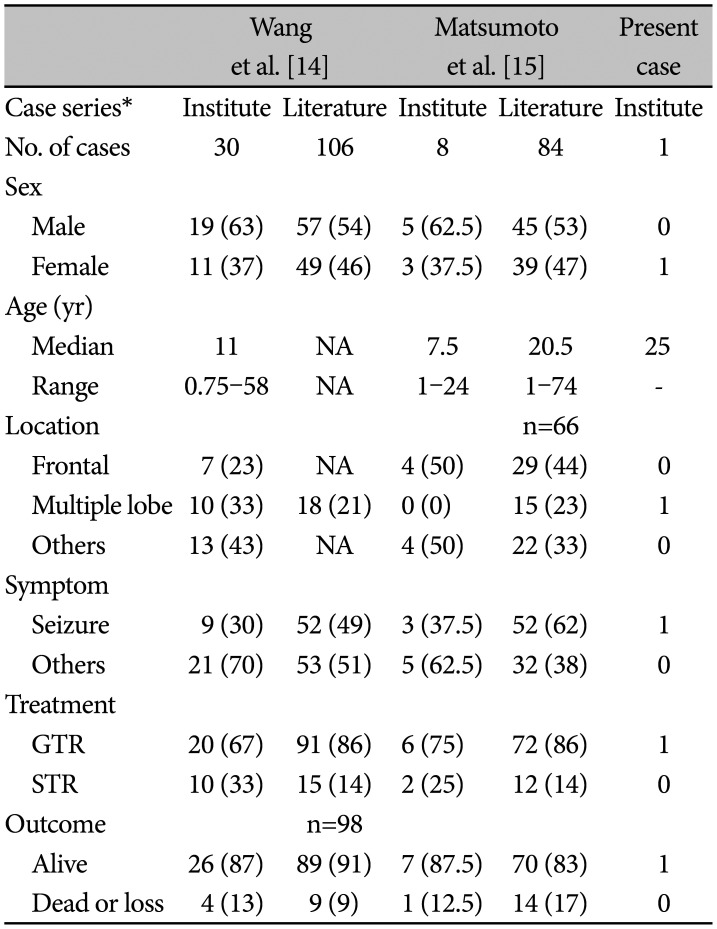
| Wang et al. [14] | Matsumoto et al. [15] | Present case | ||||
|---|---|---|---|---|---|---|
| Case series* | Institute | Literature | Institute | Literature | Institute | |
| No. of cases | 30 | 106 | 8 | 84 | 1 | |
| Sex | ||||||
| Male | 19 (63) | 57 (54) | 5 (62.5) | 45 (53) | 0 | |
| Female | 11 (37) | 49 (46) | 3 (37.5) | 39 (47) | 1 | |
| Age (yr) | ||||||
| Median | 11 | NA | 7.5 | 20.5 | 25 | |
| Range | 0.75–58 | NA | 1–24 | 1–74 | - | |
| Location | n=66 | |||||
| Frontal | 7 (23) | NA | 4 (50) | 29 (44) | 0 | |
| Multiple lobe | 10 (33) | 18 (21) | 0 (0) | 15 (23) | 1 | |
| Others | 13 (43) | NA | 4 (50) | 22 (33) | 0 | |
| Symptom | ||||||
| Seizure | 9 (30) | 52 (49) | 3 (37.5) | 52 (62) | 1 | |
| Others | 21 (70) | 53 (51) | 5 (62.5) | 32 (38) | 0 | |
| Treatment | ||||||
| GTR | 20 (67) | 91 (86) | 6 (75) | 72 (86) | 1 | |
| STR | 10 (33) | 15 (14) | 2 (25) | 12 (14) | 0 | |
| Outcome | n=98 | |||||
| Alive | 26 (87) | 89 (91) | 7 (87.5) | 70 (83) | 1 | |
| Dead or loss | 4 (13) | 9 (9) | 1 (12.5) | 14 (17) | 0 | |
Table 2
Comparisons of present case with own cases of other groups
| Wang et al. [14] | Matsumoto et al. [15] | Present case | ||
|---|---|---|---|---|
| No. of cases | 30 | 8 | 1 | |
| WHO grade | ||||
| II | 17 (57) | 1 (12.5) | 1 | |
| III | 13 (43) | 7 (87.5) | 0 | |
| Presence of cyst | ||||
| Yes | 17 (57) | 8 (100) | 0 | |
| No | 13 (43) | 0 (0) | 1 | |
| RELA-fusion* | ||||
| Yes | 9 (90) | 5 (100) | 1 | |
| No | 1 (10) | 0 (0) | 0 | |
| L1CAM stain† | ||||
| Positive | NA | 6 (75) | 1 | |
| Negative | NA | 2 (25) | 0 | |
| Recurrence | ||||
| Yes | 9 (30) | 4 (50) | 0 | |
| No | 21 (70) | 4 (50) | 1 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download