1. Little KM, Friedman AH, Sampson JH, Wanibuchi M, Fukushima T. Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery. 2005; 56:546–559. PMID:
15730581.
2. Sekhar LN, Swamy NK, Jaiswal V, Rubinstein E, Hirsch WE Jr, Wright DC. Surgical excision of meningiomas involving the clivus: preoperative and intraoperative features as predictors of postoperative functional deterioration. J Neurosurg. 1994; 81:860–868. PMID:
7965116.
3. Kim JW, Jung HW, Kim YH, et al. Petroclival meningiomas: long-term outcomes of multimodal treatments and management strategies based on 30 years of experience at a single institution. J Neurosurg. 2019; 132:1675–1682.
4. Flannery TJ, Kano H, Lunsford LD, et al. Long-term control of petroclival meningiomas through radiosurgery. J Neurosurg. 2010; 112:957–964. PMID:
19731986.
5. Roche PH, Pellet W, Fuentes S, Thomassin JM, Régis J. Gamma knife radiosurgical management of petroclival meningiomas results and in dications. Acta Neurochir (Wien). 2003; 145:883–888. PMID:
14577010.
6. Sadik ZHA, Lie ST, Leenstra S, Hanssens PEJ. Volumetric changes and clinical outcome for petroclival meningiomas after primary treatment with Gamma Knife radiosurgery. J Neurosurg. 2018; 129:1623–1629. PMID:
29372884.
7. Starke R, Kano H, Ding D, et al. Stereotactic radiosurgery of petroclival meningiomas: a multicenter study. J Neurooncol. 2014; 119:169–176. PMID:
24821284.
8. Subach BR, Lunsford LD, Kondziolka D, Maitz AH, Flickinger JC. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998; 42:437–443. discussion 443-5. PMID:
9526975.
9. Han MS, Jang WY, Moon KS, et al. Is fractionated Gamma Knife radiosurgery a safe and effective treatment approach for large-volume (>10 cm
3) intracranial meningiomas? World Neurosurg. 2017; 99:477–483. PMID:
28017757.
10. Zentner J, Meyer B, Vieweg U, Herberhold C, Schramm J. Petroclival meningiomas: is radical resection always the best option? J Neurol Neurosurg Psychiatry. 1997; 62:341–345. PMID:
9120445.
11. Bernard F, Troude L, Isnard S, et al. Long term surgical results of 154 petroclival meningiomas: a retrospective multicenter study. Neurochirurgie. 2019; 65:55–62. PMID:
31104846.
12. Nanda A, Javalkar V, Banerjee AD. Petroclival meningiomas: study on outcomes, complications and recurrence rates. J Neurosurg. 2011; 114:1268–1277. PMID:
21184632.
13. Park CK, Jung HW, Kim JE, Paek SH, Kim DG. The selection of the optimal therapeutic strategy for petroclival meningiomas. Surg Neurol. 2006; 66:160–165. discussion 165-6. PMID:
16876612.
14. Bledsoe JM, Link MJ, Stafford SL, Park PJ, Pollock BE. Radiosurgery for large-volume (>10 cm
3) benign meningiomas. J Neurosurg. 2010; 112:951–956. PMID:
19764829.
15. Chang JH, Chang JW, Choi JY, Park YG, Chung SS. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatry. 2003; 74:226–230. PMID:
12531956.
16. DiBiase SJ, Kwok Y, Yovino S, et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004; 60:1515–1519. PMID:
15590183.
17. Eustacchio S, Trummer M, Fuchs I, Schröttner O, Sutter B, Pendl G. Preservation of cranial nerve function following Gamma Knife radiosurgery for benign skull base meningiomas: experience in 121 patients with follow-up of 5 to 9.8 years. Acta Neurochir Suppl. 2002; 84:71–76. PMID:
12379007.
18. Faramand A, Kano H, Niranjan A, et al. Cranial nerve outcomes after primary stereotactic radiosurgery for symptomatic skull base meningiomas. J Neurooncol. 2018; 139:341–348. PMID:
29691775.
19. Kondziolka D, Patel AD, Kano H, Flickinger JC, Lunsford LD. Long-term outcomes after Gamma Knife radiosurgery for meningiomas. Am J Clin Oncol. 2016; 39:453–457. PMID:
24755664.
20. Starke RM, Williams BJ, Hiles C, Nguyen JH, Elsharkawy MY, Sheehan JP. Gamma knife surgery for skull base meningiomas. J Neurosurg. 2012; 116:588–597. PMID:
22175723.
21. Kim JW, Kim DG, Se YB, et al. Gamma Knife radiosurgery for petroclival meningioma: long-term outcome and failure pattern. Stereotact Funct Neurosurg. 2017; 95:209–215. PMID:
28683438.
22. Natarajan SK, Sekhar LN, Schessel D, Morita A. Petroclival meningiomas: multimodality treatment and outcomes at long-term follow-up. Neurosurgery. 2007; 60:965–979. PMID:
17538370.
23. Lynch JC, Ferreira LA, Welling L, Schulz RC. Multiple intracranial meningiomas: diagnosis, biological behavior and treatment. Arq Neuropsiquiatr. 2008; 66:702–707. PMID:
18949266.
24. Wong RH, Wong AK, Vick N, Farhat HI. Natural history of multiple meningiomas. Surg Neurol Int. 2013; 4:71. PMID:
23776757.
25. Koh YC, Yoo H, Whang GC, Kwon OK, Park HI. Multiple meningiomas of different pathological features: case report. J Clin Neurosci. 2001; 8 Suppl 1:40–43.
26. Chung LK, Mathur I, Lagman C, et al. Stereotactic radiosurgery versus fractionated stereotactic radiotherapy in benign meningioma. J Clin Neurosci. 2017; 36:1–5. PMID:
27815026.
27. Joshi KC, Raghavan A, Muhsen B, et al. Fractionated Gamma Knife radiosurgery for skull base meningiomas: a single-institution experience. Neurosurg Focus. 2019; 46:E8.
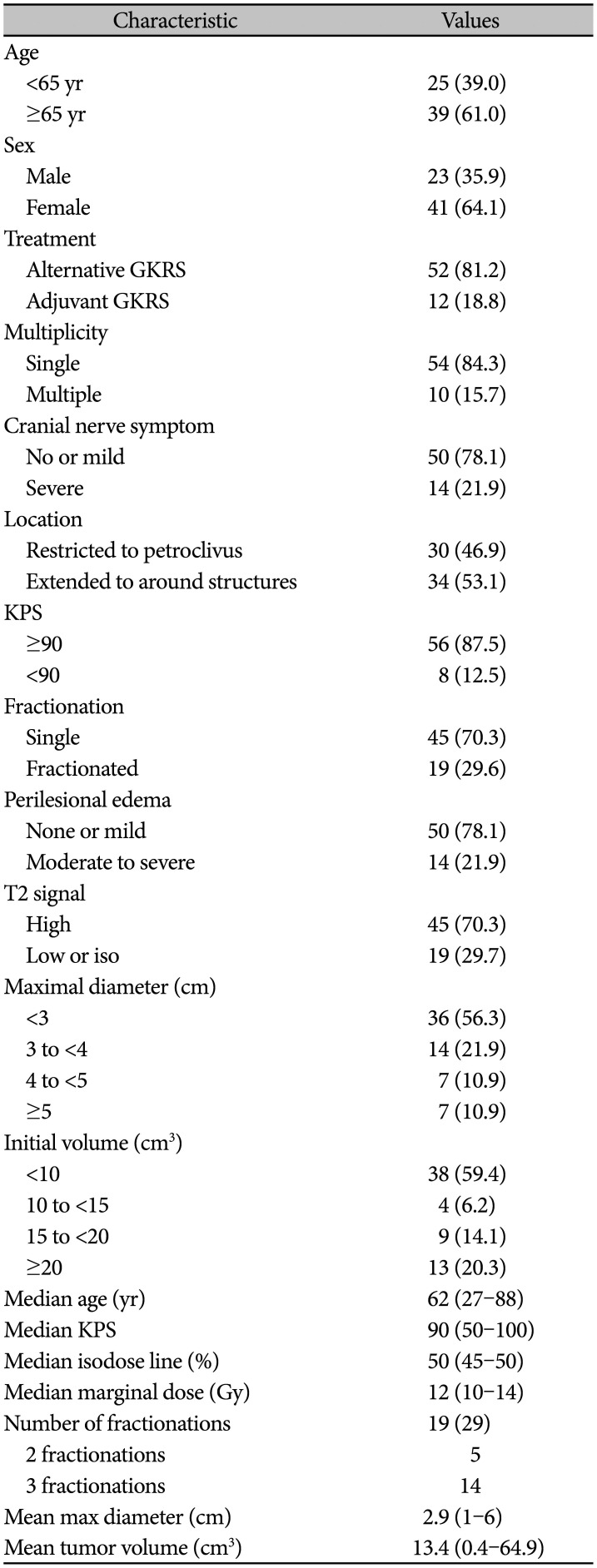
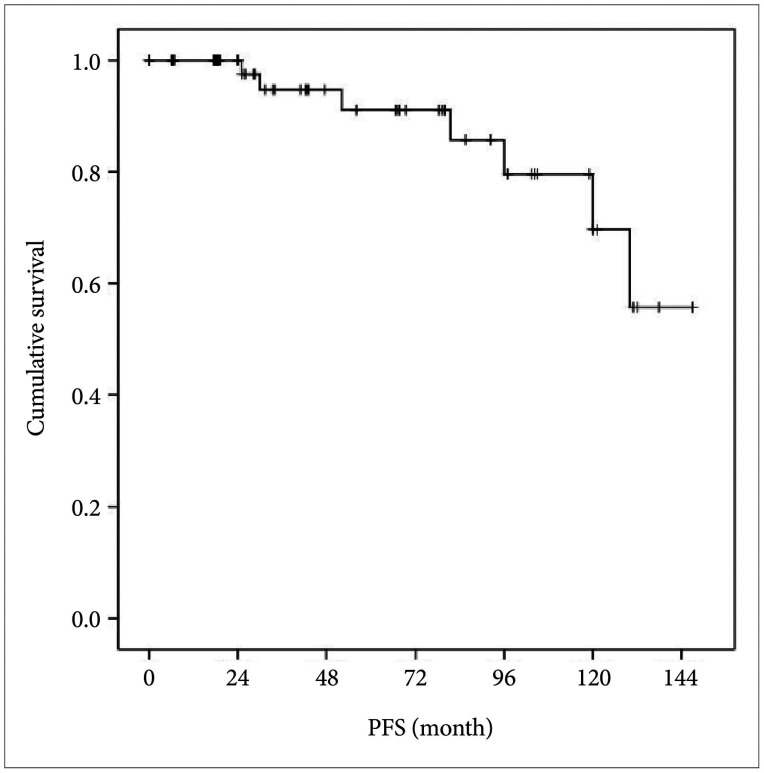
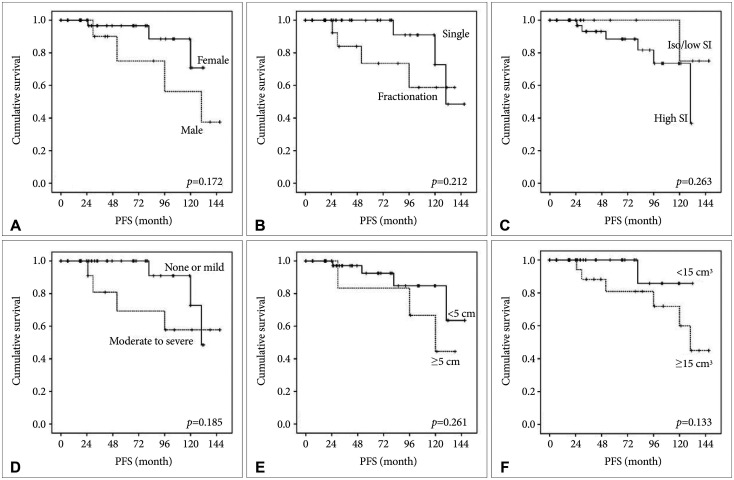
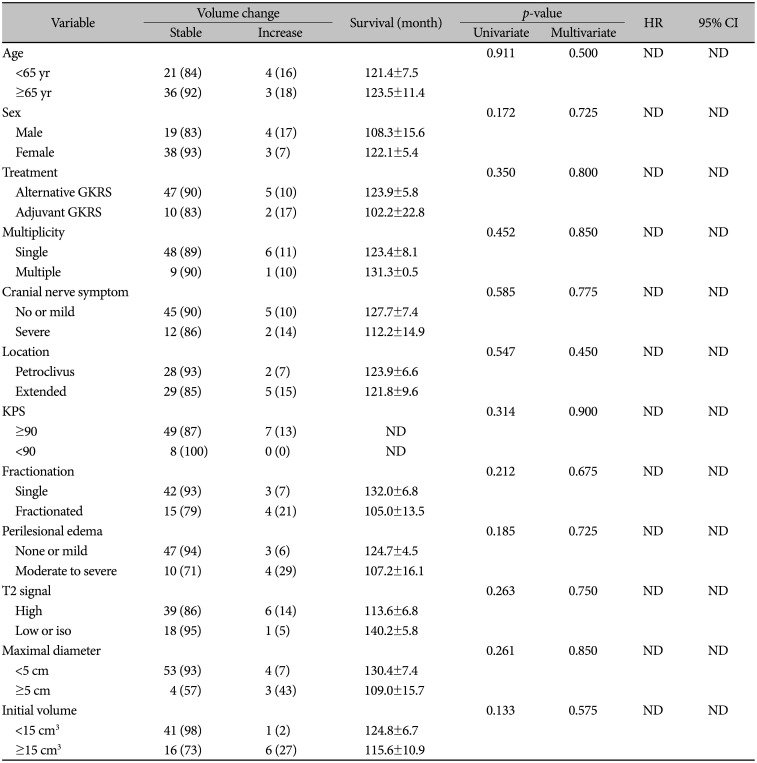




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download