Abstract
Background
High-grade glioma (HGG) with primary leptomeningeal seeding (PLS) at initial diagnosis is rare. The purpose of this study was to identify its clinical features and to describe the clinical treatment outcomes.
Methods
We retrospectively reviewed the medical records of patients with HGG (World Health Organization grade III or IV) at our institution between 2004 and 2019, and patients with PLS at the initial diagnosis were enrolled in the study. Clinical features, such as the location of leptomeningeal seeding, surgical methods, and degree of resection, were sorted based on electronic medical records also containing performance scale, and hematological and serological evaluations. Radiological findings and immunohistochemical categories were confirmed. Furthermore, we sought to determine whether controlling intracranial pressure (ICP) via early cerebrospinal fluid (CSF) diversion increases overall survival (OS) after the initial diagnosis.
Results
Of the 469 patients with HGG in our institution, less than 2% had PLS at the initial diagnosis. Most patients suffered from headache, diplopia, and dizziness. Pathological findings included 7 glioblastomas and 2 anaplastic astrocytomas. Seven of the 9 patients underwent CSF diversion. All patients were administered concurrent chemoradiotherapy (CCRT) with temozolomide, 89% of which started adjuvant temozolomide and 33% of which completed the six cycles of adjuvant temozolomide. The OS of patients with HGG and PLS was 8.7 months (range, 4-37), an extremely poor result compared to that of other studies. Also, the 1-year and 2-year OS rates were 44.4% and 16.7%, respectively.
Conclusion
Diagnosis and treatment of HGG with PLS are challenging. Aggressive control of ICP followed by early initiation of standard CCRT seems to be helpful in improving symptoms. However, despite aggressive treatment, the prognosis is poor. A multicenter trial and research may be necessary to create a standardized protocol for this disease.
Primary diffuse leptomeningeal gliomatosis (PDLG) within high-grade glioma (HGG) is a rare and serious condition. HGGs are either World Health Organization (WHO) grade III or IV tumors and they tend to grow rapidly and spread faster than tumors of lower grades. The most common grade III tumor is anaplastic astrocytoma (AA) and grade IV tumor is glioblastoma multiforme (GBM). There is a lack of consensus on the appropriate treatment for PDLG; most treatment regimens mainly focus on chemoradiotherapy and palliative management [123]. PDLG is typically characterized by glial cell infiltration with or without specific evidence of a primary brain or spinal cord mass lesion. It is clinically characterized by a rapid and progressive course, and the outcome is invariably fatal.
PDLG theoretically invades the meninges along the neural network within white matter, and it can be seeded into the subarachnoid space. Certain cases presented in this study appeared to have mass lesions displayed by pre-operative MRI, and thus do not strictly conform to the definition of PDLG. Therefore, we defined it as HGG (i.e., WHO grade III or IV) with primary leptomeningeal seeding (PLS) upon initial diagnosis.
This study enrolled patients with HGG and PLS who met the inclusion criteria; cases of secondary leptomeningeal seeding (LMS) were excluded. Of the 469 patients with HGG, 35% of which accorded with the tumors of WHO grade III such as AA, anaplastic oligodendroglioma, anaplastic ganglioma, etc., 65% of whole group were categorized in WHO grade IV like glioblastoma. Nine of these patients with PLS in present study were selected from the brain tumor database that collected information from a single center between 2004 and 2019. The chosen cases had tumor seeding at the time of the initial diagnosis based on the MRI findings. This was confirmed using radiological images and an assessment of the cerebrospinal fluid (CSF). All the selected patients underwent a biopsy.
The collected data included demographic variables, tumor characteristics, diagnostic results, treatment type and outcome, and survival after the initial diagnosis. The survival time from the initial diagnosis until death, the time of the patient's first surgery, the time of any additional surgeries for controlling increased intracranial pressure (ICP), and the administration of concurrent chemoradiotherapy (CCRT) were also evaluated.
Our baseline evaluations included a detailed patient history, physical and neurological examinations, Karnofsky Performance Scale (KPS) index, hematological and serological evaluations, and brain MRI results extracted from the electronic medical records. The Institutional Review Board (IRB) of the Seoul National University Bundang Hospital (SNUBH) waived the requirement for informed consent due to the retrospective study design, and the study was conducted according to the guidelines of the Declaration of Helsinki for biomedical research (IRB B-2010-640-107).
The treatment modalities were as follows: the first procedure involved a surgical tissue confirmation with or without cytoreduction; the second process was the CSF diversion through Ommaya reservoir insertion, extraventricular drainage (EVD) or ventriculoperitoneal (VP) shunt placement if required; the third treatment involved CCRT according to the Stupp protocol for glioblastoma.
The enrolled patients were continually followed up and monitored for signs and symptoms of increased ICP, such as headache, vomiting, and loss of consciousness. The patients presenting with these symptoms underwent EVD and VP shunt placement. In contrast, Ommaya reservoir insertion was performed as a second step in the first surgery, in which the lesion was biopsied in advance. However, it was not performed in the additional surgical procedures, as it might have interfered with early CCRT.
In this study, patients received CCRT within 6 weeks after the pathological confirmation of GBM and AA. They were administered temozolomide (TMZ) at 75 mg/m2/day for 6 weeks during CCRT based on the Stupp protocol. After a 4-week break, the patients received adjuvant TMZ for the first 5 days of each of the 28-day cycles. The number of cycles was dependent upon their situations, provided they showed signs of drug tolerance. Seven patients received additional craniospinal irradiation (CSI) for 1–4 weeks, which was also rely on the conditions.
Typical findings from MRI with gadolinium contrast (Gd) in LMS cases include an abnormal, diffuse leptomeningeal thickening and Gd enhancement at multiple sites, including the spinal cord [2]. Analysis of the CSF may show a marked increase in protein level, normal or low glucose levels, lymphocytosis, and an elevated pressure. However, staining for malignant cells reveals a negative result [6].
We conducted an MRI scan of the brain and the spinal cord at baseline and during follow-up to evaluate the primary seeding lesion. All patients with a normal CSF Gram stain result underwent a single lumbar puncture before the main surgery.
The extent of resection was evaluated using postoperative brain MRI with Gd enhancement within 48 hours after surgery. Tumor progression was evaluated using a series of MRI scans taken after surgery, during the 3rd and 6th cycles of adjuvant chemotherapy, and every 3 to 4 months thereafter. The purpose of this was to initially determine the dose of radiotherapy 4 weeks after CCRT with TMZ.
The treatment responses of the patients were categorized into four groups using the Response Assessment in Neuro-Oncology (RANO) criteria, published in 2010. It is roughly similar to other systems. It designates four types of response based on MRI and clinical features: complete response, partial response (PR), stable disease, and progressive disease. The pathologic results were determined according to the 2016 World Health Organization classification of tumors of the central nervous system. They were affirmed based on combined phenotypic and genotypic classifications and on the generation of integrated diagnoses, such as the mutation of isocitrate dehydrogenase-1 (IDH-1), status of the 1p19q co-deletion, and the methylation status of the O6-methylguanine-DNA methyltransferase (MGMT) promoter.
Of the 469 patients with HGG, 9 (1.92%) were found to have PLS at the initial diagnosis. The clinical findings in these cases are summarized in Table 1 and Supplementary Fig. 1 (in the online-only Data Supplement). The age at diagnosis ranged from 17 to 64 years (median, 41 years). Of the 9 patients, there were 8 men and 1 woman. Symptoms at onset included headache (67%), diplopia (22%), and dizziness (22%). A decrease was observed in the KPS scores after the diagnosis of PLS.
Among the 9 patients, 7 were diagnosed with GBM and 2 were identified as AA. Analyses of MGMT methylation, IDH mutations, and 1p19q co-deletion resulted in the diagnosis of GBM in the remaining 7 patients. Among the 7 GBM patients, 5 were IDH-1 wild type and one was IDH-1 mutant; 4 had an unmethylated and 3 had a methylated MGMT promotor. There were no 1p19q co-deletions among them. However, there was no results from genetic analysis for AA. Statistical analyses of the two groups (methylated vs. unmethylated) were difficult owing to the small sample size. Eight patients started adjuvant TMZ and 3 completed the six cycles of adjuvant TMZ (median value of duration of adjuvant TMZ: 4 cycles). The duration of CCRT was 41.4 days on average as seen in Table 2.
Leptomeningeal enhancement (LME) was observed on the preoperative T1-Gd MRI of the brain and spinal cord in all cases. Initially, CSF was obtained via lumbar puncture in all patients. Six patients had negative cytology results for malignancy. However, this result is not considered essential for the confirmation of PLS [78]. HGG with PLS had already been diagnosed in these patients based on the clinical and radiographic findings. The results of CSF diversion served as a baseline for monitoring the response of the patients to the treatment administered.
Surgery was performed with tissue diagnosis and ICP control as the major objectives. After pathological confirmation, CCRT was administered within 6 weeks according to the Stupp regimen. The details of the treatment are summarized in Table 2.
Seven patients had undergone ICP control surgeries, such as Ommaya reservoir insertion, EVD, and VP shunt insertion, within an estimated median of 11 days after the diagnosis of HGG with PLS. They had an average survival rate of approximately 14.2 months.
All 9 patients underwent surgery within 11 days of the LMS diagnosis based on the preoperative MRI results. Five patients underwent endoscopic biopsies and 4 underwent craniotomies with a biopsy taken. All patients completed the CCRT with TMZ as shown in Table 2.
Median overall survival (OS) was 8.7 months, and the 1-year and 2-year OS rates were 44.4% and 16.7%, respectively. According to the RANO criteria, only 1 case showed PR, and the remaining 8 patients displayed signs of disease progression within 3 years.
Only 4 patients were initially diagnosed with GBM with LMS. However, 3 patients were diagnosed with tuberculous encephalitis based on nonspecific enhancing lesions on the MRI scans and the inflammatory profile of the CSF. This can be attributed to the high incidence of tuberculosis (TB) in South Korea. Consequently, the final diagnosis in these patients took longer (25 days), compared to that of the other patients (4 days), thus leading to a delay in the surgeries.
Moreover, one of the 4 patients who underwent VP shunt insertion as an additional operation showed signs of extracranial metastases in the shoulders, pelvic bone, and femurs.
Due to the small sample size, a comparison was difficult and the prognosis of the majority of patients was poor. Thus, the statistical differences could not be evaluated. However, following the diagnosis, there was a subjective improvement in symptoms such as headache and double vision, with controlled ICP. Two patients received delayed treatment because of bone marrow suppression. Three patients switched therapy and continued treatment with Avastin® (bevacizumab)/irinotecan for 2–7 cycles. This can be attributed to an ineffectiveness of treatment based on the Stupp protocol.
This was a novel study that enrolled patients with HGG with PLS in a particular institute. At the time of this review, there were 8 deceased patients among the original 9. The OS of HGG with PLS was significantly less than 20.2 months as reported by SNUBH between 2004 and 2013 for newly diagnosed GBM (Table 3). This is based on unpublished data by the coauthor Kyeong-O Go.
In our study, CSF diversion did not increase life expectancy. However, it helped in alleviating pain. Nine patients completed CCRT without any significant side-effects. No adverse effects were associated with TMZ administration during CSI. Thus, the patients with LMS of the spine required active treatment of their spinal cavity.
There is a high incidence of TB encephalitis in Korea. Patients initially misdiagnosed with TB meningitis or encephalitis, with symptoms consisting of convulsions or headache and LME displayed via MRI without specific findings upon CSF examination, received TB treatment for more than 2 weeks in our study. However, there was no improvement of symptoms, upon which those patients were referred to the neurosurgery department for the possibility of the presence of a tumor. In these cases, the neurologists and radiologists did not suspect GBM with LMS, which our study has revealed as a possibility. Therefore, a tissue biopsy should be performed to identify ill-defined lesions of the brain parenchyma associated with clinical symptoms of encephalitis, with a high index of suspicion for high-grade tumors. This specimen can be obtained by stereotactic or navigation-guided biopsy.
Systemic metastasis to extracranial sites is extremely rare, occurring at an estimated incidence of less than 2% [910]. Nonetheless, one of our patients who underwent VP shunting displayed signs of extracranial metastases to the shoulders, pelvic bones, and femurs.
Furthermore, there have been numerous reports of postoperative and peritoneal metastases following the implantation of a VP shunt. This suggests that metastases can occur because of intraoperative manipulation and the displacement of malignant cells into the blood, CSF, and lymphatic systems [111213]. Thus, it would be advisable to explore a method that prevents metastases to other organs after rapid ICP control and before chemotherapy.
Though we could not compare the effectiveness of EVD, VPS, and Ommaya insertion for CSF diversion because of the small sample size within our study, it might prove beneficial to control ICP at the beginning and during the suspension of aggressive tumor seeding to other organs.
The diagnosis of and treatment for PDLG are challenging. A 10-year study on GBM conducted at the SNUBH between 2004 and 2011 had a median OS of 19.3 months. In addition, the 1-year and 2-year OS rates were 78.3% and 41.7%, respectively [10]. The results of our study indicate that patients initially diagnosed with HGG with PLS have a poor prognosis, and the OS rate is considerably lower than that of glioblastoma without LMS. Our study demonstrates a difference of around 5 months. Thus, the early initiation of standard CCRT immediately after CSF diversion might improve quality of life by alleviating symptoms such as pain.
Despite the lack of consensus on the optimal management of HGG with PLS, such treatments as stereotactic biopsy, cranial radiotherapy, and tumor resection should aim at preventing further CSF dissemination. Maximal resection is the initial therapy of choice for intracranial GBM. It frequently results in a rapid improvement in symptoms and associated survival rates. Preventive radiotherapy of the entire craniospinal axis might also prove effective.
In conclusion, considering that patients with LMS in this study had worsening symptoms and a poor prognosis, it is necessary to implement chemoradiotherapy at an early stage and more aggressively than in treatment implemented for GBM. This study did not reveal significant differences between the effectiveness of different types of CSF diversion owing to the small sample size. In addition, future research should include a multi-center study to create a standardized protocol for HGG with PLS management.
References
1. Schwartz C, Romagna A, Machegger L, et al. Extensive leptomeningeal intracranial and spinal metastases in a patient with a supratentorial glioblastoma multiforme, IDH-wildtype. World Neurosurg. 2018; 120:442–447. PMID: 30253992.
2. Sulentic V, Hajnsek S, Petelin Gadze Z, Bujan Kovac A, Nankovic S. Primary diffuse leptomeningeal gliomatosis: early diagnostic signs. Neurol Sci. 2015; 36:1697–1699. PMID: 25904056.
3. Jicha GA, Glantz J, Clarke MJ, et al. Primary diffuse leptomeningeal gliomatosis. Eur Neurol. 2009; 62:16–22. PMID: 19407451.
4. Kim BS, Seol HJ, Nam DH, et al. Concurrent chemoradiotherapy with temozolomide followed by adjuvant temozolomide for newly diagnosed glioblastoma patients: a retrospective multicenter observation study in Korea. Cancer Res Treat. 2017; 49:193–203. PMID: 27384161.
5. Noh JH, Lee MH, Kim WS, et al. Optimal treatment of leptomeningeal spread in glioblastoma: analysis of risk factors and outcome. Acta Neurochir (Wien). 2015; 157:569–576. PMID: 25663100.
6. Mandel JJ, Yust-Katz S, Cachia D, et al. Leptomeningeal dissemination in glioblastoma; an inspection of risk factors, treatment, and outcomes at a single institution. J Neurooncol. 2014; 120:597–605. PMID: 25168214.
7. Shakur SF, Bit-Ivan E, Watkin WG, Merrell RT, Farhat HI. Multifocal and multicentric glioblastoma with leptomeningeal gliomatosis: a case report and review of the literature. Case Rep Med. 2013; 2013:132679. PMID: 24381594.
8. Waki F, Ando M, Takashima A, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol. 2009; 93:205–212. PMID: 19043775.
9. Kim H, Lim DH, Kim TG, et al. Leptomeningeal enhancement on preoperative brain MRI in patients with glioblastoma and its clinical impact. Asia Pac J Clin Oncol. 2018; 14:e366–e373. PMID: 29473335.
10. Joo JD, Kim H, Kim YH, Han JH, Kim CY. Validation of the effectiveness and safety of temozolomide during and after radiotherapy for newly diagnosed glioblastomas: 10-year experience of a single institution. J Korean Med Sci. 2015; 30:1597–1603. PMID: 26539003.
11. Cho HJ, Myung JK, Kim H, et al. Primary diffuse leptomeningeal glioneuronal tumors. Brain Tumor Pathol. 2015; 32:49–55. PMID: 24770606.
12. Bernardini FP, Croxatto JO, Nozza P, Rossi A, Capris P. Primary diffuse leptomeningeal gliomatosis in children: a clinical pathologic correlation. Ophthalmic Plast Reconstr Surg. 2013; 29:93–97. PMID: 23247038.
13. Lawton CD, Nagasawa DT, Yang I, Fessler RG, Smith ZA. Leptomeningeal spinal metastases from glioblastoma multiforme: treatment and management of an uncommon manifestation of disease. J Neurosurg Spine. 2012; 17:438–448. PMID: 22958073.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14791/btrt.2020.8.e18.
Supplementary Fig. 1
Preoperative MRI findings of nine patients (A–I), presented in Table 1 in order. The suspicious lesions are indicated by arrows (F and H).
Table 1
Summary of high grade gliomas patients with primary leptomeningeal seeding at initial diagnosis (9 cases)
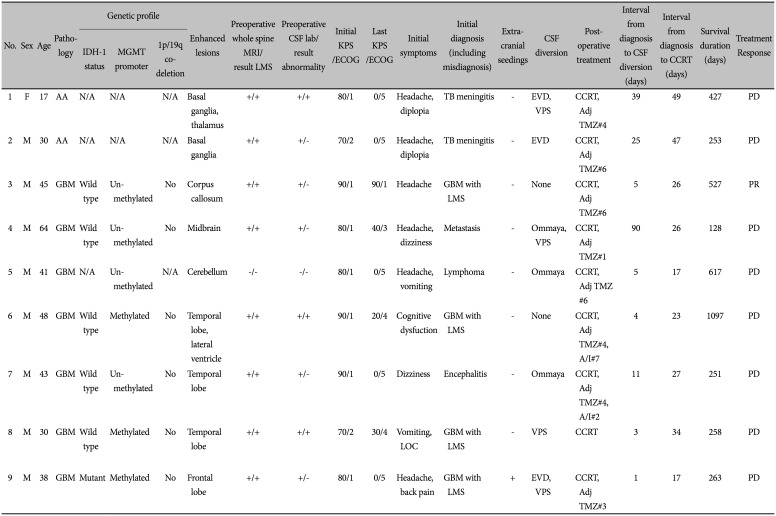
AA, anaplastic astrocytoma; GBM, glioblastoma multiforme; IDH-1, isocitrate dehydrogenase-1; MGMT, O6-methylguanine-DNA methyltransferase; LMS, leptomeningeal seeding; CSF, cerebrospinal fluid; KPS, Karnofsky Performance Scale; ECOG, Eastern Cooperative Oncology Group performance status score; LOC, loss of consciousness; TB, tuberculosis; EVD, extraventricular drainage; VPS, ventriculoperitoneal shunt; PD, progression disease; PR, partial response; CCRT, concurrent chemoradiotherapy; Adj TMZ, adjuvant temozolomide (#=cycle); A/I, Avastin® (bevacizumab)/irinotecan
Table 2
Treatment details and intensities
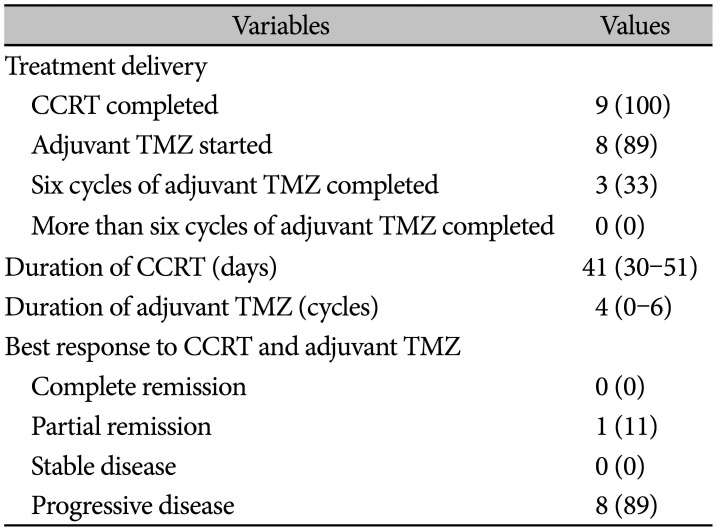
Table 3
Kaplan-Meier estimates of overall survival (OS) of the 9 high-grade glioma (HGG) with primary leptomeningeal seeding (PLS) patients in the present study compared to that of the 112 newly diagnosed GBM patients in the same institute
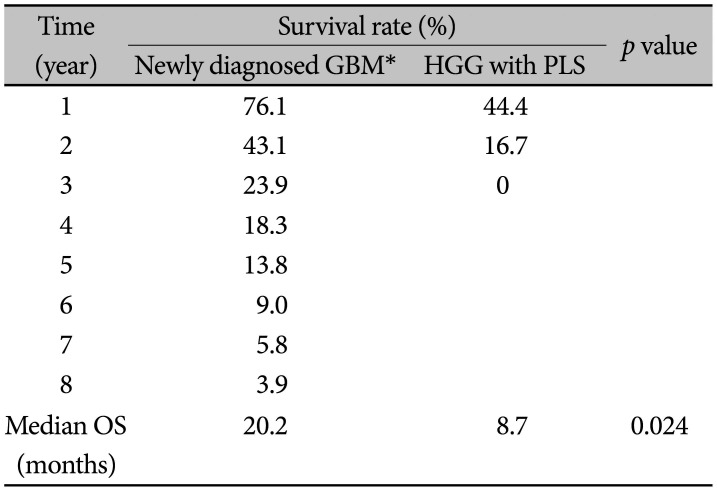
| Time (year) | Survival rate (%) | p value | |
|---|---|---|---|
| Newly diagnosed GBM* | HGG with PLS | ||
| 1 | 76.1 | 44.4 | |
| 2 | 43.1 | 16.7 | |
| 3 | 23.9 | 0 | |
| 4 | 18.3 | ||
| 5 | 13.8 | ||
| 6 | 9.0 | ||
| 7 | 5.8 | ||
| 8 | 3.9 | ||
| Median OS (months) | 20.2 | 8.7 | 0.024 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download