1. Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009; 69:5383–5391. PMID:
19549917.
2. Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011; 17:5473–5480. PMID:
21737504.
3. Mendez JS, Govindan A, Leong J, Gao F, Huang J, Campian JL. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol. 2016; 127:329–335. PMID:
26725885.
4. Campian JL, Piotrowski AF, Ye X, et al. Serial changes in lymphocyte subsets in patients with newly diagnosed high grade astrocytomas treated with standard radiation and temozolomide. J Neurooncol. 2017; 135:343–351. PMID:
28756593.
5. Ahn S, Park JS, Jang J, et al. The association between total lymphocyte count after concomitant chemoradiation and overall survival in patients with newly diagnosed glioblastoma. J Clin Neurosci. 2020; 71:21–25. PMID:
31843432.
6. Kim WJ, Dho YS, Ock CY, et al. Clinical observation of lymphopenia in patients with newly diagnosed glioblastoma. J Neurooncol. 2019; 143:321–328. PMID:
30982199.
7. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674. PMID:
21376230.
8. Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012; 12:269–281. PMID:
22437939.
9. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018; 24:1459–1468. PMID:
30104766.
10. Grassberger C, Ellsworth SG, Wilks MQ, Keane FK, Loeffler JS. Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol. 2019; 16:729–745. PMID:
31243334.
11. Huang J, DeWees TA, Badiyan SN, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015; 92:1000–1007. PMID:
26025775.
12. Rodríguez DM, Guerrero ME, Maldonado BM, Vollbracht C, Herrera SA. Total lymphocyte count in cancer patients with lymphopenia treated with intravenous vitamin C: results of an observational study. Transl Med Commu. 2017; 2:3.
13. Chitadze G, Flüh C, Quabius ES, et al. In-depth immunophenotyping of patients with glioblastoma multiforme: impact of steroid treatment. Oncoimmunology. 2017; 6:e1358839. PMID:
29147621.
14. Rudra S, Hui C, Rao YJ, et al. Effect of radiation treatment volume reduction on lymphopenia in patients receiving chemoradiotherapy for glioblastoma. Int J Radiat Oncol Biol Phys. 2018; 101:217–225. PMID:
29502931.
15. Lin AJ, Campian JL, Hui C, et al. Impact of concurrent versus adjuvant chemotherapy on the severity and duration of lymphopenia in glioma patients treated with radiation therapy. J Neurooncol. 2018; 136:403–411. PMID:
29143923.
16. Ye LL, Fan XW, Hu CS, et al. Dosimetry of the brain and hypothalamus predicting acute lymphopenia and the survival of glioma patients with postoperative radiotherapy. Cancer Med. 2019; 8:2759–2768. PMID:
30983159.
17. Hui CY, Rudra S, Ma S, Campian JL, Huang J. Impact of overall corticosteroid exposure during chemoradiotherapy on lymphopenia and survival of glioblastoma patients. J Neurooncol. 2019; 143:129–136. PMID:
30864102.
18. Byun HK, Kim N, Yoon HI, et al. Clinical predictors of radiation-induced lymphopenia in patients receiving chemoradiation for glioblastoma: clinical usefulness of intensity-modulated radiotherapy in the immuno-oncology era. Radiat Oncol. 2019; 14:51. PMID:
30917849.
19. Piotrowski AF, Nirschl TR, Velarde E, et al. Systemic depletion of lymphocytes following focal radiation to the brain in a murine model. Oncoimmunology. 2018; 7:e1445951. PMID:
29900062.
20. Wong ET, Lok E, Gautam S, Swanson KD. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br J Cancer. 2015; 113:1642.
21. Pitter KL, Tamagno I, Alikhanyan K, et al. Corticosteroids compromise survival in glioblastoma. Brain. 2016; 139:1458–1471. PMID:
27020328.
22. Cenciarini M, Valentino M, Belia S, et al. Dexamethasone in glioblastoma multiforme therapy: mechanisms and controversies. Front Mol Neurosci. 2019; 12:65. PMID:
30983966.
23. Sampson JH, Maus MV, June CH. Immunotherapy for brain tumors. J Clin Oncol. 2017; 35:2450–2456. PMID:
28640704.
24. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001; 95:190–198.
25. Ahn S, Park JS, Song JH, Jeun SS, Hong YK. Effect of a time delay for concomitant chemoradiation after surgery for newly diagnosed glioblastoma: a single-institution study with subgroup analysis according to the extent of tumor resection. World Neurosurg. 2020; 133:e640–e645. PMID:
31568907.
26. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011; 335:2–13. PMID:
20398732.
27. Purton JF, Monk JA, Liddicoat DR, et al. Expression of the glucocorticoid receptor from the 1A promoter correlates with T lymphocyte sensitivity to glucocorticoid-induced cell death. J Immunol. 2004; 173:3816–3824. PMID:
15356129.
28. Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci. 2006; 63:60–72. PMID:
16314919.
29. Wüst S, van den, Tischner D, et al. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J Immunol. 2008; 180:8434–8443. PMID:
18523311.
30. Gustafson MP, Lin Y, New KC, et al. Systemic immune suppression in glioblastoma: the interplay between CD14
+HLA-DR
lo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010; 12:631–644. PMID:
20179016.
31. Shields LB, Shelton BJ, Shearer AJ, et al. Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat Oncol. 2015; 10:222. PMID:
26520780.
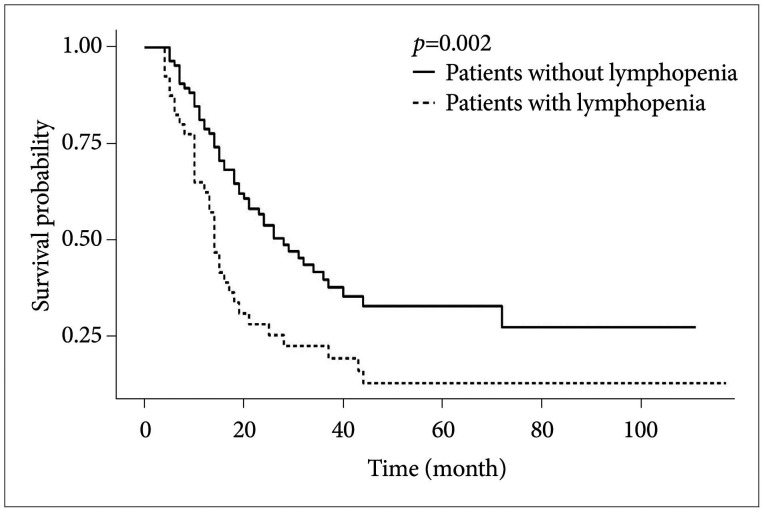
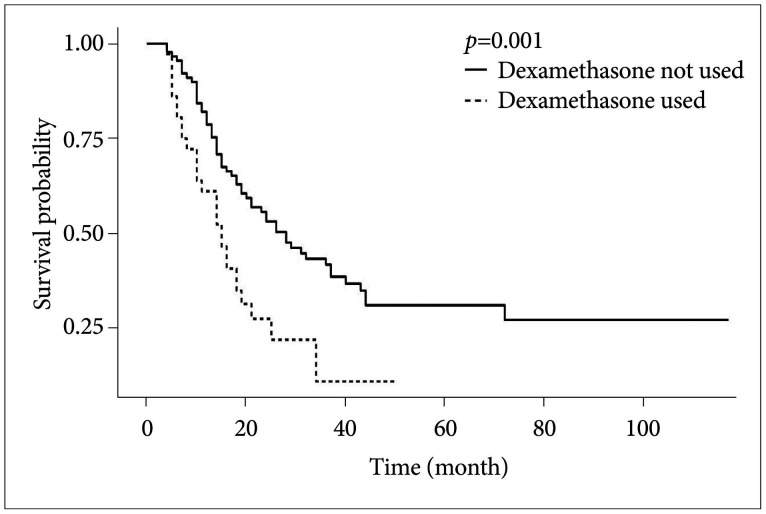
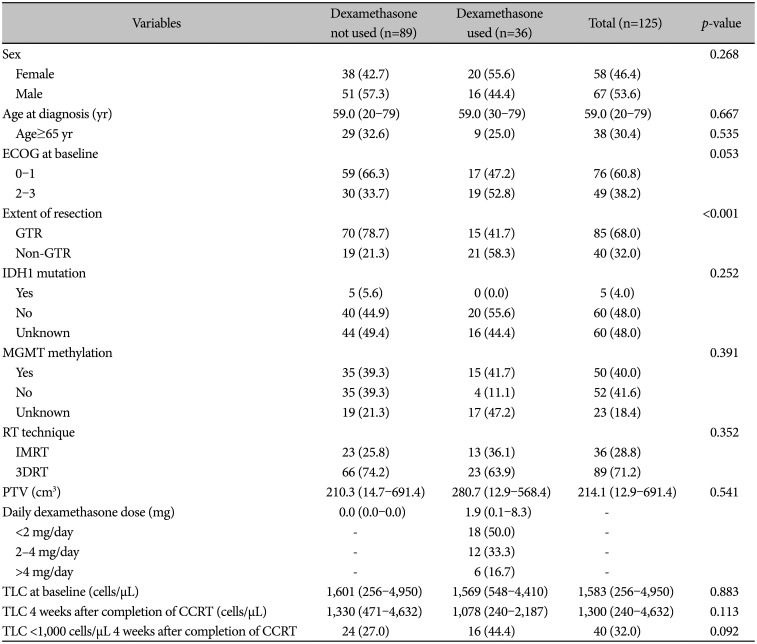
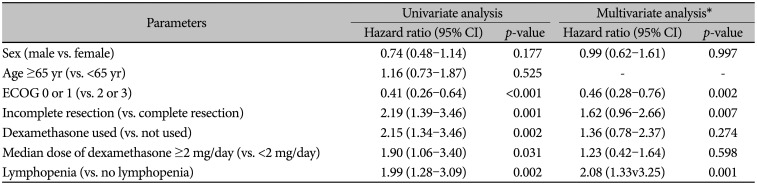




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download