Abstract
The vast majority of intracranial chondromas are located in the base of the cranium. Their presentation as an intracerebral neoplasm is considered to be extremely rare. A previously healthy 45-year-old man experienced recurrent seizure attacks over a period of 6 months. Imaging studies of the brain revealed an ovoid and calcified mass which involve the cortex in the left frontal lobe. Intraoperatively, the mass had no adhesion to the dura and arachnoid membrane. The tumor was completely removed and the final diagnosis was intracerebral chondroma. The patient remained free of disease over the period of 18-years follow-up. This communication adds an unusual case to the literature on the chondroma that expressed in the form of an intra-axial space-occupying lesion in the frontal lobe.
Intracranial chondromas arises from the rests of embryonic cartilage along the sphenopetrosal and sphenooccipital synchondroses at the base of the skull [1]. These chondromas are benign, slow-growing neoplasms, presenting about 0.2–0.3% of all primary intracranial tumors [2]. They are most frequently identified at an extradural location in the parasellar region or in the paranasal sinuses, with bulging into the intracranial cavity. In rare occasions, they could be particularly found in the subdural space around the convexity and falx cerebri [34].
Chondroma which occurs within the cerebrum without meningeal involvement is named intracerebral chondroma. To the best of our knowledge, only small numbers of intraparenchymal types of chondroma have been documented previously [56]. In this report, the author illustrates a special case of chondroma which located in the left frontal lobe. A review of relevant literature is also provided for the purpose of gaining more knowledge about an ectopic location of this rare cartilaginous tumor.
A 45-year-old gentleman was referred to the emergency department with 6-month history of episodic convulsions. On admission, neurological examinations did not reveal any focal defects or signs. He had no remarkable medical history. All laboratory investigations were within normal range. CT from another hospital showed a solitary, mixed density mass with calcification in the frontal lobe. MR images revealed a cortical tumor which was hypointense on T1-weighted image (T1WI) and centrally hyperintense with a hypointense periphery on T2-weighted images (T2WI). A slight rim enhancement was seen after gadolinium injection (Fig. 1A–D). According to the clinical history and radiological findings, this patient was preliminary diagnosed as a calcified meningioma.
A rectangular frontal bone flap was elevated to approach the mass. Dura mater was opened and a solid extra-axial tumor was exposed. The tumor was not attached to the dura mater. Microscopically, the arachnoid was intact, and it separated the dura and the tumor surface. Tumor was clearly isolated from adjacent parenchyma and could be extirpated in an en bloc. The patient tolerated the operation with an uneventful hospital course. The patient has been followed-up for more than 18 years. He lives in good condition with no tumor recurrence (Fig. 1E).
The surgical specimen was 23×18×15 mm in size, creamy-white, and lobulated. Light microscopic examination demonstrated the globular arrangement of well-differentiated chondrocytes situated within the lacuna. On immunohistochemistry, tumors stained positive for vimentin and S-100 protein. The histopathologic study confirmed the diagnosis of chondroma (Fig. 2).
The IRB exempted informed consent due to its retrospective nature and minimal risk for harm to the patient, and this report was conducted according to the guidelines of the Declaration of Helsinki for biomedical research.
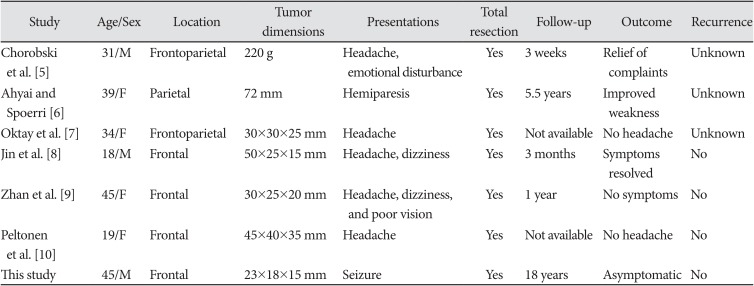
Chondromas generally develop from the basal skull, whereas their presence at the cerebral cortex is exceptional. There are very few reports on the truly parenchymal lesions in the related literatures [78]. The authors compiled the clinical and radiological features of the published cases along with the current one. The frontal region is the most common localization of the intracerebral chondroma in the previously reported cases (Table 1) [910]. The studies revealed that cranial chondromas had no sex predominance and commonly manifested in the group of younger ages [1112].
Explanations for the histogenesis of chondromas at the meninges or within the parenchyma have been postulated. Meningeal or intraventricular tumors most likely originated from cartilaginous metaplasia of the fibroblast in the falx, convexity dura or ependymal lining [1314]. The proliferation of ectopic embryologic chondrocytes or transformation of perivascular mesenchymal tissue was proposed as a theory for development of intracerebral chondroma [91516]. Intracranial chondromas usually appear as an isolated mass; however, sometimes they can be associated with systemic chondromatosis condition, such as Ollier's disease or Mafucci's syndrome [161718].
Morphologically, cranial chondromas are characterized by their expansive and irregular growth pattern, compared to those occurring at other sites [19]. On pathological examination, intracranial chondromas commonly appeared as mature cartilage in the lobules formed of neoplastic chondrocyte resembles normal cells. The tumor is frequently calcified and ossified in its center. The biopsy sample of the present case also had the vascular axes within the tumor that is an exclusive feature of chondromas [20]. In occasion, chondromas are difficult to distinguish morphologically from chordomas, but positive immunohistochemical stains for epithelial membrane antigen, S-100 protein, cytokeratin and vimentin suggest the diagnosis of chordoma [221]. It is not surprising that hypercellularity and nuclear polymorphism are observed in the histological study of multiple chondromatosis. Additionally, it is well recognized that patients with disease-related chondromas are at increased risk of malignant degeneration and secondary chondrosarcoma [18].
Since cranial chondromas have slow-growing nature, these tumors may have a long asymptomatic period [22]. In many cases, they have reached an advanced tumor size at the time of diagnosis [41119]. Symptomatology is not specific and mostly depends on the anatomic location and extension of the lesions. Most of patients with basal chondromas had symptoms of intracranial hypertension, seizure, focal neurological sign or cranial neuropathy [2323]. The clinical features of parenchymal type of chondromas are somehow similar to meningiomas, even though they occur more often in the younger population. Authors noted that the published chondromas with intraparenchymal location commonly produced headache, personality change, convulsion, dizziness, visual blurring, and limb weakness [12345].
In head CT scans, most cranial chondromas appear as a mass of mixed density with minimal to moderate enhancement. The rare types have a less dense area due to mucous degeneration at the center of the cartilaginous tumor [24]. With their rarity, however, basal chondroma might be confused with craniopharyngioma, chordoma, or even cerebral aneurysm on the preoperative studies. Dynamic CT shows delayed contrast enhancement, which is related to the phenomenon of less feeders and angiogenesis within this cartilaginous neoplasm [25]. This feature can be employed in differential diagnosis of chondromas from meningiomas and scwannomas. MRI reveals a well demarcated mass without peritumoral edema in the reported chondromas, as in our patient. These tumors exhibit heterogeneous signal intensities, hypointense on T1WI and asymmetric signal on T2WI in the not calcified portions. The region with calcifications is hypointense on both T1- and T2-weighted sequences. A slight to moderate annular enhancement can be seen at the fibrous capsule in the periphery of the tumor [11121]. Notwithstanding, the clinical symptoms and imaging findings are not characteristic, and intracerebral chondroma tends to misdiagnose to glioma, cavernoma, granuloma, or hematoma.
Little is known about the natural history of this cranial cartilaginous neoplasm with benign characteristics. Complete surgical removal of the tumors will restore the clinical deficits in almost all patients [923]. Surgery of chondromas is not difficult because of the avascularity of the tumor and lack of invasion into the surrounding structures [419]. Complete resection of tumors is the best mode of treatment, especially in cases of intracerebral type with clear margin such as ours. After complete removal of these tumors, no recurrence has been reported and a satisfactory long-term prognosis was obtained [3812]. Rarely, there have been recurrences in the cranial chondromas which partially extirpated, leading to malignant disintegration into chondrosarcoma [20]. Moreover, researchers concluded that radiation may initiate the process of malignant degeneration within tumors, so radiotherapy is not recommended for the cases with non-operable or residual chondromas [11].
In summary, the presented report highlights that the brain parenchyme is a rare location of occurrence for intracranial chondroma. This chondroma cannot be preoperatively differentiated from other intra-axial tumors based solely on the clinical presentations and neuroimaging characteristics. Total tumor excision is the curative therapy for the patients with intracerebral chondromas.
References
1. Sullivan JC, Goldsmith J, Rojas R, Varma H, Kasper EM. Intracranial dural parafalcine chondroma: case report and systematic review of the literature. World Neurosurg. 2019; 122:1–7. PMID: 30273721.
2. Weng JC, Li D, Li H, et al. Surgical management and outcomes of intracranial chondromas: a single-center case series of 66 patients. World Neurosurg. 2017; 108:264–277. PMID: 28867324.
3. Fountas KN, Stamatiou S, Barbanis S, Kourtopoulos H. Intracranial falx chondroma: literature review and a case report. Clin Neurol Neurosurg. 2008; 110:8–13. PMID: 17913345.
4. Nakayama M, Nagayama T, Hirano H, Oyoshi T, Kuratsu J. Giant chondroma arising from the dura mater of the convexity. Case report and review of the literature. J Neurosurg. 2001; 94:331–334. PMID: 11213975.
5. Chorobski J, Jarzymski J, Ferens E. Intracranial solitary chondroma. Surg Gynecol Obstet. 1939; 68:677–686.
6. Ahyai A, Spoerri O. Intracerebral chondroma. Surg Neurol. 1979; 11:431–433. PMID: 483149.
7. Oktay K, Dere UA, Arslan M, Kesen S, Ciftci T. Intracranial chondroma without meningeal attachment. Neurol India. 2018; 66:865–866. PMID: 29766963.
8. Jin Y, Zhang X, Ge J, Zhang S, Liu Q, Qiu Y. Chondroma located in cerebral parenchyma without meningeal attachment. J Craniofac Surg. 2012; 23:1910–1912. PMID: 23172440.
9. Zhan RY, Pan XF, Wan S, et al. Solitary intracerebral chondroma without meningeal attachment: a case report with review of the literature. J Int Med Res. 2011; 39:675–681. PMID: 21672374.
10. Peltonen E, Suess O, Koenneker M, Brock M, Kombos T. Atypical location of a solitary intracranial chondroma without meningeal attachment. Zentralbl Neurochir. 2007; 68:83–86. PMID: 17614089.
11. Geng S, Zhang J, Zhang LW, et al. Diagnosis and microsurgical treatment of chondromas and chondrosarcomas of the cranial base. Oncol Lett. 2014; 8:301–304. PMID: 24959265.
12. Abeloos L, Maris C, Salmon I, Balériaux D, Sadeghi N, Lefranc F. Chondroma of the dural convexity: a case report and literature review. Neuropathology. 2012; 32:306–310. PMID: 22017366.
13. Hirvonen J, Heikinheimo H. A case of intracerebral chondroma. A case report. Acta Pathol Microbiol Scand. 1969; 76:19–24. PMID: 5350748.
14. Feierabend D, Maksoud S, Lawson McLean A, Koch A, Kalff R, Walter J. Giant convexity chondroma with meningeal attachment. Clin Neurol Neurosurg. 2018; 169:37–40. PMID: 29609117.
15. Hong JT, Lee SW, Son BC, Sung JH, Choi HC, Kim MC. Delayed occurrence of intracranial supratentorial chondroma following compound depressed skull fracture. Acta Neurochir (Wien). 2005; 147:343–345. discussion 345. PMID: 15605200.
16. Tibbs RE, Bowles AP, Raila FA. Maffucci's syndrome and intracranial chondrosarcoma. Skull Base Surg. 1997; 7:49–55. PMID: 17171008.
17. Nakase H, Nagata K, Yonezawa T, Morimoto T, Sakaki T. Extensive parasellar chondroma with Ollier's disease. Acta Neurochir (Wien). 1998; 140:100–101. PMID: 9522917.
18. Xin Y, Hao S, Zhang J, et al. Microsurgical treatment of intracranial chondroma. J Clin Neurosci. 2011; 18:1064–1071. PMID: 21719289.
19. Rosenberg AE, Nielsen GP, Keel SB, et al. Chondrosarcoma of the base of the skull: a clinicopathologic study of 200 cases with emphasis on its distinction from chordoma. Am J Surg Pathol. 1999; 23:1370–1378. PMID: 10555005.
20. Colpan E, Attar A, Erekul S, Arasil E. Convexity dural chondroma: a case report and review of the literature. J Clin Neurosci. 2003; 10:106–108. PMID: 12464537.
21. Schmidinger A, Rosahl SK, Vorkapic P, Samii M. Natural history of chondroid skull base lesions--case report and review. Neuroradiology. 2002; 44:268–271. PMID: 11942386.
22. Cherekaev VA, Golbin DA, Gasparyan TG, Shishkina LV, Tsukanova TV. Management of craniofacial chondroid tumors. J Craniofac Surg. 2015; 26:10–18. PMID: 25569383.
23. Abdelhamid K, Camras LR, Nijensohn EM, Rosseau GL, Cerullo LJ. Intracranial chondroma arising from the cranial vault: CT and MR appearance. J Comput Assist Tomogr. 1996; 20:556–558. PMID: 8708055.
24. Duan F, Qiu S, Jiang J, et al. Characteristic CT and MRI findings of intracranial chondroma. Acta Radiol. 2012; 53:1146–1154. PMID: 22983260.
25. Tanohata K, Maehara T, Aida N, et al. Computed tomography of intracranial chondroma with emphasis on delayed contrast enhancement. J Comput Assist Tomogr. 1987; 11:820–823. PMID: 3655044.
Fig. 1
MR images reveal an oval-shaped mass which involve the cortex in the left frontal lobe. A: T2-weighted image depicts an intracerebral mass with a hypointense periphery and relatively hyperintense central core. B–D: It is hypointense to gray matter and rim-enhanced on T1-weighted images. E: There is no evidence of tumor recurrence on the enhanced CT scan taken 18 years later after the surgery.
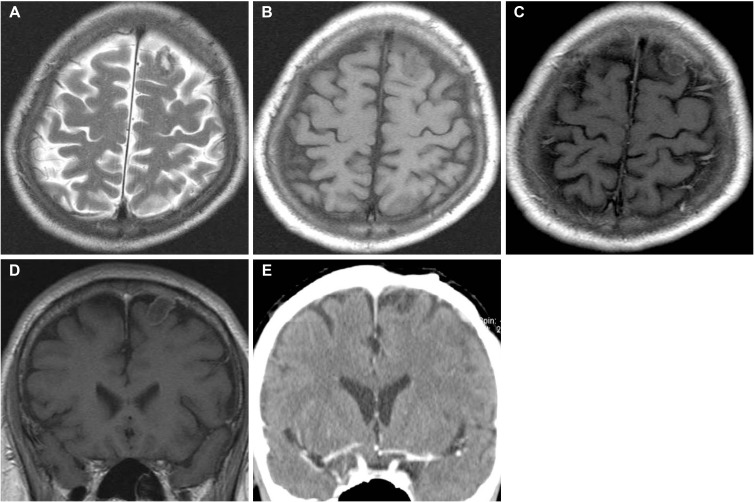
Fig. 2
Light microscopy of the tumor displays a gray-blue cartilage matrix and mature chondrocytes (hematoxylin and eosin staining, ×200).
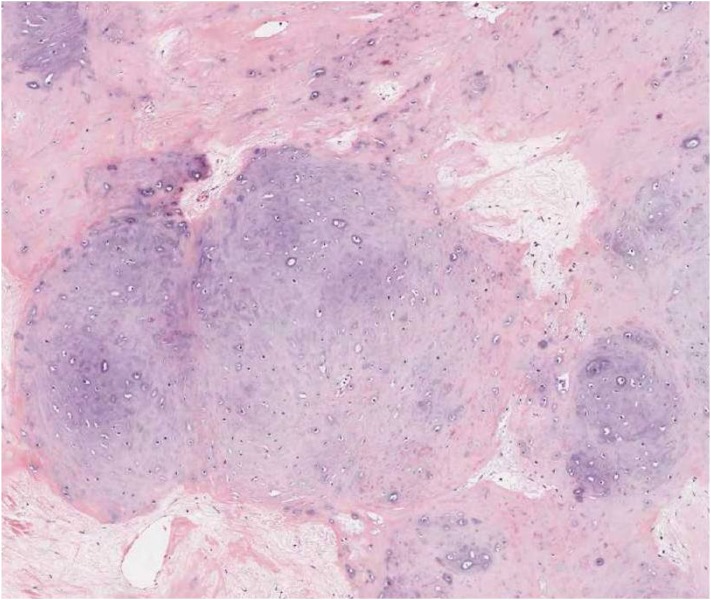
Table 1
Summary of the cases with intracerebral chondroma in the literature
| Study | Age/Sex | Location | Tumor dimensions | Presentations | Total resection | Follow-up | Outcome | Recurrence |
|---|---|---|---|---|---|---|---|---|
| Chorobski et al. [5] | 31/M | Frontoparietal | 220 g | Headache, emotional disturbance | Yes | 3 weeks | Relief of complaints | Unknown |
| Ahyai and Spoerri [6] | 39/F | Parietal | 72 mm | Hemiparesis | Yes | 5.5 years | Improved weakness | Unknown |
| Oktay et al. [7] | 34/F | Frontoparietal | 30×30×25 mm | Headache | Yes | Not available | No headache | Unknown |
| Jin et al. [8] | 18/M | Frontal | 50×25×15 mm | Headache, dizziness | Yes | 3 months | Symptoms resolved | No |
| Zhan et al. [9] | 45/F | Frontal | 30×25×20 mm | Headache, dizziness, and poor vision | Yes | 1 year | No symptoms | No |
| Peltonen et al. [10] | 19/F | Frontal | 45×40×35 mm | Headache | Yes | Not available | No headache | No |
| This study | 45/M | Frontal | 23×18×15 mm | Seizure | Yes | 18 years | Asymptomatic | No |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download