Abstract
Sinonasal teratocarcinosarcoma (SNTCS), a very rare tumor, is known to be a heterogeneous with epithelial, mesenchymal, and neuroepithelial components and shows a very aggressive clinical course. Due to the heterogeneity of this tumor, it is often misdiagnosed. No definitive treatment modality has been reported because it is a very rare tumor. A 44-year-old man presented to a rhinologist with headache and nasal obstruction, and an intranasal tumor was found that invaded into the cranial cavity. He underwent combined surgery with a rhinologist and a neurosurgeon following cognitive decline that worsened after a transnasal biopsy. The patient was diagnosed with SNTCS and underwent radiotherapy. However, residual tumor was found during radiotherapy and additional chemotherapy was administered. Follow-up brain MRI revealed no remnant or recurrent lesion. SNTCS is a tumor that has not yet been well researched and should be further investigated for proper treatment.
A large tumor involving both the anterior cranial fossa and the nasal cavity is rarely encountered by a neurosurgeon, and a differential diagnosis based on radiological and histological findings is needed. Due to its anatomical location, multidisciplinary approaches are required for surgical resection as well as adjuvant treatment. Among the tumors that develop in the nasal cavity and invade into the cranial cavity, sinonasal teratocarcinosarcoma (SNTCS) is a very rare tumor characterized by a heterogenous pathology [12345678]. There are less than 100 cases reported in English journals, and intracranial extension is present in about 20.9% of cases [3]. We report surgical experience of a large SNTCS with an intracranial extension.
A 44-year-old man presented with an aggravating headache for 1 month. He had no past medical history. Neurologic examination showed cognition decline (Mini-Mental State Examination score, 20) and a slightly drowsy mentality. MRI with gadolinium enhancement (GdE) showed a large (9 cm in diameter) dumbbell-shaped mass that occupied both the anterior cranial fossa and the nasal cavity with strong heterogeneous enhancement and perilesional brain edema (Fig. 1). A brain CT scan showed bilateral erosion of cribriform plates (Fig. 2). The initial preliminary diagnosis was olfactory neuroblastoma, and transnasal endoscopic biopsy was done by a rhinologist (Fig. 3). The pathologic diagnosis was neuroendocrine carcinoma. Therefore, a whole body positron emission tomography CT and chest-abdomen CT was performed, but no specific findings were found in other sites.
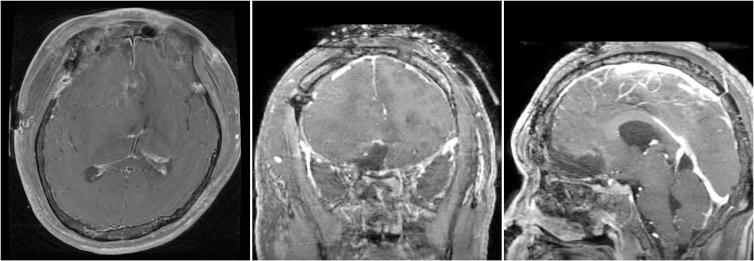
The patient underwent a combined operation by a neurosurgeon and rhinologist. First, the neurosurgeon removed the intracranial tumor with a right subfrontal approach. The tumor tissue was firm to friable with a highly developed vasculature and a reddish-purple appearance. Because of the highly developed vasculature, bleeding was severe during the operation. After the intracranial mass was removed, a small defect in the ethmoid bone could be seen at the skull base. However, the defect was so small we did not perform skull base reconstruction, but instead added multiple layers of a fibrin sealant patch (tachosil). Following the gross total resection of the intracranial mass, the rhinologist performed transnasal endoscopic resection, and the mass was totally removed. One day after the operation, brain MRI with GdE showed the tumor was totally resected with no residual lesion (Fig. 4).
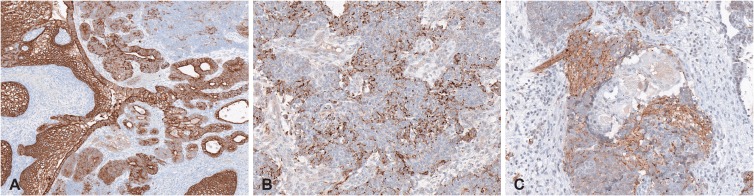
The pathologic finding indicated a tumor with a solid growth pattern and hemorrhage. The tumor was composed of epithelial and mesenchymal components in a neurofibrillary background (Fig. 5). The epithelial component consisted of stratified squamous epithelium with clear cell change and adenocarcinoma. The epithelial component was surrounded by immature neuroepithelial cells with a primitive blastema-like feature. The mesenchymal component consisted of malignant spindle cells with a myxoid stroma. From the immunohistochemical stain, the epithelial component showed strong positivity for cytokeratin (Fig. 6). The neuroepithelial cells were positive for chromogranin and synaptophysin. The final diagnosis was SNTCS.
The cognitive function of the patient improved after the operation. Postoperation 6 weeks later, adjuvant radiation therapy (60 Gy in 30 fractions) was administered. At the end of radiation therapy, a biopsy was performed in the otolaryngology outpatient clinic on the granulation tissue in the nasal cavity, and the pathologic report revealed recurred or remnant SNTCS. After radiation therapy, chemotherapy (AI protocol: doxorubicin+ifosfamide) was added and it is currently in process. Nine months after the surgery, a 7th chemotherapy has been completed. Brain MRI performed twice at 4 and 9 months after operation revealed no remnant or recurrent lesion.
The nasal cavity is an adjacent space below the skull base, but it is uncommon for tumors originating here to invade the cranial cavity. These tumors are often found by otorhinolaryngologists due to symptoms such as nasal congestion and are sometimes referred to neurosurgeons for consultation. SNTCS is an uncommon and extremely malignant neoplasm that is characterized by a combination of histological features that include both teratoma and carcinosarcoma, but without malignant germ cell components [1]. It was first described by Heffner and Hyams [2] in 1984 as a fetal-appearing squamous epithelium composed of squamous cells with clear cytoplasm. SNTCS develops most often in the nasal cavity and invades into the ethmoid sinus and the maxillary sinus. Intracranial extension is present in about 20.9% of cases [3].
Misra et al. [3] reviewed 86 cases of reported SNTCS. The most common symptom was nasal obstruction, followed by epistaxis. Further symptoms include headache, visual symptoms, and olfactory disturbance. In addition, neurological symptoms associated with the frontal lobe, such as somnolence, withdrawn affect, abnormal behavior, and confusion, have been reported. SNTCS occurs most often in males (male- to female ratio of 7:1 to 8:1) [24] with a mean age of 54.5 years. The mean survival time is less than 2 years, and the mortality rate has been reported to be about 60% within 3 years [1].
SNTCS has many tissue components associated with all 3 germ layers that are either mature or immature. Its composition includes a combination of epithelial, mesenchymal, and neuroepithelial components. The epithelial parts include keratinizing and non-keratinizing squamous epithelium, pseudostratified columnar ciliated epithelium, and glandular/ductal structures. The mesenchymal parts include fibroblasts, striated and smooth muscle, osteoblasts, cartilage, and myofibroblasts. The neuroepithelial components include a proliferation of immature round cells either in solid nests or within a neurofibrillary background with characteristics of SNTCS [15].
SNTCS is often misdiagnosed, and adequate tissue sampling is often required for accurate diagnosis. Furthermore, because this tumor shows a wide variety of pathologic features, if the amount of specimen is too small, it can sometimes be misdiagnosed [6]. In our case, the first attempted nasal biopsy obtained 2 pieces of soft tissue, which was a small sample of 0.4 cm in diameter and the initial diagnosis was misdiagnosed as neuroendocrine carcinoma. In the differential diagnosis of SNTCS, olfactory neuroblastoma and carcinosarcoma can be considered. There is a lot of epithelial differentiation in SNTCS, and the existence of mesenchymal components can be distinguished from olfactory neuroblastoma [4]. Neuroectodermal differentiation is also a prominent feature that is not present in carcinosarcoma [7].
The most common treatment modalities for SNTCS have been surgical resection and postoperative radiotherapy [367]. In 24 cases that included postoperative radiotherapy, the average dose was 54.9 Gy (range, 10 Gy to 70 Gy). In those cases, the most commonly used chemotherapeutic agent was cisplatin [3]. In one report, histology-specific multidrug chemotherapy was performed in regard to the major components of the heterogenous histology of SNTCS, where cisplatin, etoposide, and ifosfamide were given, and the patient had no recurrence in follow up [8]. In our case, doxorubicin and ifosfamide were used, as this protocol is mainly used in soft tissue sarcoma [9]. At 9 months follow up, he completed 7 cycles of chemotherapy, and currently there is no tumor recurrence, but continuous follow up is needed to confirm the treatment results. Ideal treatment strategies are difficult to identify because these cases are rare and there are no established therapies. Currently, radical surgical resection followed by radiation therapy appears to be the most commonly used treatment option. Despite the common treatment strategies (surgery and radiation therapy) currently used, recurrence occurs in 26.1% of cases, metastasis in 10.9% cases, and both metastasis and recurrence in 8.7% of cases [3].
SNTCS is known to be a very aggressive, rapidly progressing tumor with a poor prognosis. Due to its rarity and histological heterogeneity, it is often misdiagnosed, and adequate tissue sampling is required for diagnosis [1011]. The optimal management of SNTCS still has not been established. Further studies on the efficacy of adjuvant chemotherapy are warranted, and appropriate treatment should be established.
References
1. El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours. 4th ed. Lyon: International Agency for Research on Cancer;2017. p. 267.
2. Heffner DK, Hyams VJ. Teratocarcinosarcoma (malignant teratoma?) of the nasal cavity and paranasal sinuses a clinicopathologic study of 20 cases. Cancer. 1984; 53:2140–2154. PMID: 6322958.
3. Misra P, Husain Q, Svider PF, Sanghvi S, Liu JK, Eloy JA. Management of sinonasal teratocarcinosarcoma: a systematic review. Am J Otolaryngol. 2014; 35:5–11. PMID: 23731851.
4. Wei S, Carroll W, Lazenby A, Bell W, Lopez R, Said-Al-Naief N. Sinonasal teratocarcinosarcoma: report of a case with review of literature and treatment outcome. Ann Diagn Pathol. 2008; 12:415–425. PMID: 18995206.
5. Yang S, Sun R, Liang J, Zhou Z, Zhou J, Rui J. Sinonasal teratocarcinosarcoma: a clinical and pathological analysis. Int J Surg Pathol. 2013; 21:37–43. PMID: 22923779.
6. Sweety Vijay S, Kumar TD, Srikant B, Vithal SH, Vijay KS, Gurunath P. Intracranial presentation of teratocarcinosarcoma. J Clin Neurosci. 2010; 17:1347–1349. PMID: 20655234.
7. Kim JH, Maeng YH, Lee JS, Jung S, Lim SC, Lee MC. Sinonasal teratocarcinosarcoma with rhabdoid features. Pathol Int. 2011; 61:762–767. PMID: 22126386.
8. Nitsche M, Hermann RM, Christiansen H, Berger J, Pradier O. Rationale for individualized therapy in Sinonasal Teratocarcinosarcoma (SNTC): case report. Onkologie. 2005; 28:653–656. PMID: 16330889.
9. Casali PG, Abecassis N, Bauer S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018; 29 Suppl 4:iv51–iv67.
10. Vranic S, Caughron SK, Djuricic S, et al. Hamartomas, teratomas and teratocarcinosarcomas of the head and neck: report of 3 new cases with clinico-pathologic correlation, cytogenetic analysis, and review of the literature. BMC Ear Nose Throat Disord. 2008; 8:8. PMID: 19025657.
11. Carrizo F, Pineda-Daboin K, Neto AG, Luna MA. Pharyngeal teratocarcinosarcoma: review of the literature and report of two cases. Ann Diagn Pathol. 2006; 10:339–342. PMID: 17126251.
Fig. 1
Brain MRI with gadolinium enhancement shows large mass involving right nasal cavity with marked intracranial extension.

Fig. 2
Brain CT enhancement shows an intracranial extended nasal mass with erosion of cribriform plate.
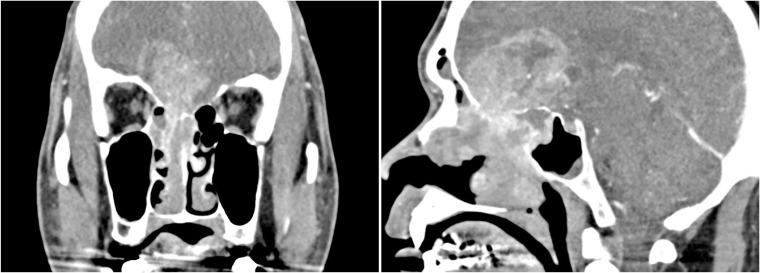
Fig. 5
Histopathological finding with hematoxylin and eosin staining. A: Microscopically, epithelial component and mesenchymal components are shown in neurofibrillary background (original magnification ×100). B: Stratified squamous epithelium with clear cell change are admixed with malignant spindle cells (original magnification ×200). C: Adenocarcinoma is surrounded by immature neuroepithelial cells (original magnification ×200). D: Malignant spindle cells with myxoid stroma about the adenocarcinoma (original magnification ×200).





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download