Abstract
Background
To compare the diagnostic performance of two-dimensional (2D) and three-dimensional (3D) fractal dimension (FD) and lacunarity features from MRI for predicting the meningioma grade.
Methods
This retrospective study included 123 meningioma patients [90 World Health Organization (WHO) grade I, 33 WHO grade II/III] with preoperative MRI including post-contrast T1-weighted imaging. The 2D and 3D FD and lacunarity parameters from the contrast-enhancing portion of the tumor were calculated. Reproducibility was assessed with the intraclass correlation coefficient. Multivariable logistic regression analysis using 2D or 3D fractal features was performed to predict the meningioma grade. The diagnostic ability of the 2D and 3D fractal models were compared.
Results
The reproducibility between observers was excellent, with intraclass correlation coefficients of 0.97, 0.95, 0.98, and 0.96 for 2D FD, 2D lacunarity, 3D FD, and 3D lacunarity, respectively. WHO grade II/III meningiomas had a higher 2D and 3D FD (p=0.003 and p<0.001, respectively) and higher 2D and 3D lacunarity (p=0.002 and p=0.006, respectively) than WHO grade I meningiomas. The 2D fractal model showed an area under the curve (AUC), accuracy, sensitivity, and specificity of 0.690 [95% confidence interval (CI) 0.581–0.799], 72.4%, 75.8%, and 64.4%, respectively. The 3D fractal model showed an AUC, accuracy, sensitivity, and specificity of 0.813 (95% CI 0.733–0.878), 82.9%, 81.8%, and 70.0%, respectively. The 3D fractal model exhibited significantly better diagnostic performance than the 2D fractal model (p<0.001).
Meningiomas are the most common primary intracranial neoplasms in adults, which account for over a third of primary intracranial neoplasms, with an incident rate of 8.03 per 100,000 population [1]. The 2016 World Health Organization (WHO) classification categorizes meningiomas into 3 grades [2]. Using modern WHO criteria, 70–75% of surgically resected meningiomas are grade I (benign), 20–30% are grade II (atypical), and 1–3% are grade III (anaplastic) [2]. Compared to benign (WHO grade I) meningiomas, atypical (WHO grade II) or anaplastic (WHO grade III) tumors have an aggressive clinical behavior, an increased risk of tumor recurrence, as well as an poor prognosis [3].
The ability to preoperatively differentiate between WHO grade I and WHO grade II/III meningiomas has crucial clinical ramifications. Patients with WHO grade II/III meningiomas could benefit from early and complete resection [4]. Moreover, according to the European Association of Neuro-Oncology guideline, incidentally discovered menigiomas on MRI can be managed by observation [5]; thus, the histological grade may not be confirmed. Thus, noninvasive prediction of the meningioma grade may support clinical decision-making by providing information on whether observation should be performed. However, currently, the value of meningioma grading on the basis of neuroimaging has been low [6]. Conventional imaging features are subjective [7], and diffusion and perfusion imaging has shown contradictory results [8910].
Fractal features are image descriptors that mathematically measures the geometrical complexity [1112]. In particular, the uniqueness of fractal features lies in its ability to quantify natural objects with high structural complexities that are poorly represented by the conventional Euclidean geometry, thereby allowing analysis of underlying tissue biology reflected as morphological information. Fractal dimension (FD) and lacunarity are the two parameters used in fractal analysis. FD describes the intrinsic shape of an object; as the FD increases, the complexity increases [12]. Lacunarity is a geometric measure that represents the degree of rotational (or translational) invariance and gappiness [12]. Several studies have performed fractal analysis on differentiating various brain tumors with promising results [131415], but most studies have analyzed only two-dimensional (2D) or three-dimensional (3D) fractal features. By encompassing the entire tumor, 3D fractal features may potentially provide a more comprehensive assessment of the fractal properties of a tumor and show better diagnostic performance than 2D fractal features. However, there has been no previous study that compared the diagnostic accuracy of 2D and 3D fractal models.
We hypothesized that 3D fractal features may exhibit true fractal behavior and show superiority in grading meningioma than 2D fractal features. Thus, the purpose of this study was to compare the diagnostic performance of 2D and 3D FD and lacunarity features for predicting the grade of meningiomas.
This retrospective study was approved by the Yonsei University Heath System Institutional Review Board, and a waiver of informed consent was obtained. One hundred thirty-one meningioma cases in which pathological confirmation and preoperative conventional MRI were performed between June 2009 and January 2018 were included in this study. Exclusion criteria were as follows: 1) patients with a past history of brain surgery (n=6), and 2) patients who have undergone tumor embolization or gamma knife surgery before MRI examination (n=2). A total of 123 patients were included in this study (100 females and 23 males; mean age: 57.9±13.0 years).
MRI scans was performed using a 3-T MRI scanner (Achieva, Philips Medical Systems, Best, the Netherlands) with an eight-channel head coil. The preoperative MRI protocol included T1-weighted [repetition time (TR)/echo time (TE): 2,000/10 ms; field of view: 230 mm; section thickness: 5 mm; matrix: 320×198] and post-contrast T1-weighted (T1C) images (TR/TE: 2000/10 ms; field of view: 250 mm; section thickness: 2 mm; and matrix: 256×256). T1C images were acquired after the administration of 0.1 mL/kg of gadolinium-based contrast material (Gadovist; Bayer, Leverkusen, Germany).
According to the 2016 WHO criteria, pathological diagnosis and meningioma grading was performed by a neuropathologist [12]. The mitosis count was evaluated using the mitotic marker phosphohistone-H3. The mitosis index was determined by counting the number of unequivocal mitotic figures in 10 consecutive high-power fields (×400) containing the highest number of mitoses. The Ki-67 labeling index was estimated.
Tumor segmentation was performed by a neuroradiologist (Y.W.P.), blinded to the clinical and histopathologic results. The regions of interests were segmented using an open source software platform for medical image informatics (3D slicer, version 4.10.2; available at: http://slicer.org). Regions of interests were drawn on every tumor slice on T1C images, using an intensity threshold-based semiautomatic method. Gross cystic, necrotic, or hemorrhagic areas were avoided using T1-weighted and T1C images. To test the interobserver reproducibility, images from 20 patients were randomly selected and separately segmented by another neuroradiologist (S.S.A.).
Computation of the 2D and 3D box-counting FD and lacunarity values were performed using the mask after binarization [16]. FD was calculated by using a 2D square and 3D cube box-counting method, with multiple grid offsets for all possible box start locations. Lacunarity was calculated by averaging the square of coefficient of variation values of multiple 2D square and 3D cube boxes that included a part of the binary mask [17]. Since the optimal box size was unknown, both FD and lacunarity values were computed with different 2D and 3D box sizes, ranging from 20 to 27 isotropic pixels and voxels. The mean 2D and 3D FD and lacunarity values were calculated in each patient and were used in further analysis (Details of FD and lacunarity calculations are available in the Supplementary Material in the online-only Data Supplement) [16171819]. Fig. 1 shows the schematic for tumor segmentation and fractal analyses.
The baseline characteristics were compared between patients with WHO grade I and WHO grade II/III meningiomas using the independent t-test or Mann-Whitney U-test for continuous variables according to normality, and chi-square test for categorical variables. The interobserver reproducibility of fractal parameters was assessed by two-way interclass correlation.
Univariate and multivariable logistic regression analyses were performed using 2D or 3D fractal features to find the features that are significantly associated with WHO grade II/III meningiomas. Variables of interest in the univariate analysis (p<0.05) were included in the multivariable models by using the backward selection method. Because the aim of this study was to compare the diagnostic accuracy of 2D and 3D fractal models, 2D fractal models were consisted of significant 2D fractal features in the univariate results, and 3D fractal models were consisted of significant 3D fractal features in the univariate results, respectively. We estimated the area under the curve (AUC) to assess the predictive ability of the multivariable models. The diagnostic performance of the 2D fractal model was compared with that of a 3D fractal model. De-Long's method was used to compare the AUCs among the 2D fractal model and 3D fractal model [20].
All statistical analyses were performed using the MedCalc Statistical Software version 16.2.0 (https://www.medcalc.org). A p-value <0.05 was considered statistically significant.
The clinical, histopathological characteristics and fractal features of the 123 patients are summarized in Table 1. There were 90 WHO grade I meninigiomas and 33 WHO grade II/III meningiomas. Among WHO grade I meningiomas, 44 were transitional, 27 meningothelial, 10 fibroblastic, four psammomatous, two angiomatous, two microcystic, and one secretory. Among WHO grade II/III meningiomas, 29 atypical and four anaplastic meningiomas. Patients with WHO grade II/III meningiomas had a lower prevalence of skull base location than WHO grade I meningiomas (12.1% vs. 30.0%, p=0.043).
WHO grade II/III meningiomas had higher 2D FD (1.4 vs. 1.3, p=0.003), higher 2D lacunarity (2.8 vs. 2.7, p=0.002), higher 3D FD (2.0 vs. 1.9, p<0.001), and higher 3D lacunarity (5.2 vs. 4.8, p=0.006) than WHO grade I meningiomas. Fig. 2 shows the boxplots of the 2D and 3D fractal features according to different meningioma grades.
The reproducibility between observers was excellent for both 2D and 3D fractal features, with intraclass correlation coefficients of 0.97 [95% confidence interval (CI) 0.96–0.98] for 2D FD, 0.95 (95% CI 0.94–0.96) for 2D lacunarity, 0.98 (95% CI 0.97–0.99) for 3D FD, and 0.96 (95% CI 0.95–0.97) for 3D lacunarity.
On univariate analysis, higher 2D FD [odds ratio (OR)=235.8 (95% CI 5.3–10,547.9), p=0.005], higher 3D FD [OR=97.9 (95% CI 6.9–1,381.1), p=0.001], and higher 3D lacunarity [OR=2.2 (95% CI 1.2–3.8), p=0.008] were associated with WHO grade II/III meningiomas. Because the significant variable on univariate analysis was included in the 2D fractal model, 2D FD [OR=235.8 (95% CI 5.3–10,547.9), p=0.005] was the only independent variable for predicting the meningioma grade in the 2D fractal model. In multivariable analysis for 3D fractal model, 3D FD [OR=1,250.3 (95% CI 42.2–37,022.8), p<0.001] and lacunarity [OR 4.0 (95% CI 2.0–8.1), p<0.001] were independent variables for predicting the meningioma grade (Univariate and multivariable results are on Table 2).
The 2D fractal model showed an AUC, accuracy, sensitivity, and specificity of 0.690 (95% CI 0.581–0.799), 72.4%, 75.8%, and 64.4%, respectively. The 3D fractal model showed an AUC, accuracy, sensitivity, and specificity of 0.813 (95% CI 0.733–0.878), 82.9%, 81.8%, and 70.0%, respectively. In the comparison of the predictive power for WHO grade II/ III meningiomas in the two multivariable models, 3D fractal model exhibited significantly better diagnostic performance than 2D fractal model (p<0.001) (Table 3, Fig. 3).
Spatial heterogeneity is an important property displayed by tumors that has biological consequences. In this study, we comparatively analyzed the diagnostic accuracy of 2D and 3D fractal features in grading meningioma, showing that 3D fractal features show significantly better performance in grading meningioma. To the best of our knowledge, this is the first study comparing the diagnostic accuracy of 2D and 3D fractal features in medical imaging. In routine clinical practice, surgery is usually recommended for patients with mass effect or symptoms [5]. However, WHO grade II/III meningiomas patients may benefit from early operation even in the absence of these radiological or clinical findings. Our results provide a quantitative method to preoperatively differentiate WHO grade I from WHO grade II/III meningiomas, and may aid clinical decision making before treatment.
Both the 2D and 3D FD were significantly higher in WHO grade II/III meningiomas than in WHO grade I meningiomas, suggesting that WHO grade II/III meningiomas exhibit a more complex structure. Also, the lacunarity of WHO grade II/III meningiomas was significantly higher than that of WHO grade I meningiomas, suggesting that the necrosis which are visualized as gaps in the tumor lesion may increase its rotational variance. However, 2D lacunarity was not a significant predictor in the univariate analysis, and 3D fractal model showed significantly superior performance to 2D fractal model. Because meningioma exhibits a complex 3D structure, 2D fractal analysis shows limitations in that it is performed with an averaging of parameter values under a particular angle view of this complex 3D structure, which may not be fully representative of the whole tumor and supports the case for investigating 3D fractal features [2122]. In our results, 2D and 3D FD and lacunarity differed in values, and 3D fractal features provided values with larger magnitude than 2D fractal features, which reflects the volume assessed.
Previous studies have demonstrated that meningiomas with a high proliferative potential may exhibit highly heterogeneous distributions of proliferating cells in the tumor, resulting in irregular shapes [2324]. Our study provides a quantitative method to assess this complexity in grading meningioma. Some recent studies have focused on radiomics features for differentiating the meningioma grade [2526], but fractal features have not been routinely implemented in the previous radiomics studies [2627282930]. Apart from other radiomics features, fractal features are known to be relatively stable, as shown in our study as high reproducibility. Moreover, radiomics studies are prone to overfitting due to high dimensionality and high-probability of false-positive rate, and the large number of features requires intensive computational resources [31]. We used a conventional T1C sequence that is routinely included in the MRI rather than advanced imaging techniques, such as diffusion tensor imaging or amide proton transfer imaging [72632], suggesting a more feasible methodology.
Our study has several limitations. First, our study was a retrospective study based on a single institution with a limited sample size. Future studies with a larger sample is necessary for validation. Second, there is data imbalance in the WHO grade I and WHO grade II/III meningiomas. However, our proportion of meningioma grades are in concordance to the proportion reported according to the 2016 WHO classification [2], as this is an retrospective study. Third, comparison of WHO grade II and grade III meningiomas were not performed, due to small data size.
In conclusion, we compared the diagnostic performance 2D and 3D fractal features in grading meningioma. The 3D fractal analysis proved superiority in diagnostic performance to 2D fractal analysis in grading meningioma, and may be useful for treatment planning.
Acknowledgments
This research received funding from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Communication Technologies & Future Planning (2017R1D1A1B03030440). This work was supported under the framework of international cooperation program managed by National Research Foundation of Korea (NRF-2018K2A9A2A06020642). This research was supported financially by the fund of Korean Society for Neuro Oncology.
References
1. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016; 18(suppl_5):v1–v75. PMID: 28475809.
2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820. PMID: 27157931.
3. Kshettry VR, Ostrom QT, Kruchko C, Al-Mefty O, Barnett GH, Barnholtz-Sloan JS. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro Oncol. 2015; 17:1166–1173. PMID: 26008603.
4. Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015; 122:4–23. PMID: 25343186.
5. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016; 17:e383–e391. PMID: 27599143.
6. Nowosielski M, Galldiks N, Iglseder S, et al. Diagnostic challenges in meningioma. Neuro Oncol. 2017; 19:1588–1598. PMID: 28531331.
7. Joo B, Han K, Choi YS, et al. Amide proton transfer imaging for differentiation of benign and atypical meningiomas. Eur Radiol. 2018; 28:331–339. PMID: 28687916.
8. Sanverdi SE, Ozgen B, Oguz KK, et al. Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol. 2012; 81:2389–2395. PMID: 21723681.
9. Azizyan A, Eboli P, Drazin D, Mirocha J, Maya MM, Bannykh S. Differentiation of benign angiomatous and microcystic meningiomas with extensive peritumoral edema from high grade meningiomas with aid of diffusion weighted MRI. Biomed Res Int. 2014; 2014:650939. PMID: 25478572.
10. Zhang H, Rödiger LA, Shen T, Miao J, Oudkerk M. Preoperative subtyping of meningiomas by perfusion MR imaging. Neuroradiology. 2008; 50:835–840. PMID: 18542938.
11. Lee G, Lee HY, Park H, et al. Radiomics and its emerging role in lung cancer research, imaging biomarkers and clinical management: state of the art. Eur J Radiol. 2017; 86:297–307. PMID: 27638103.
12. Lennon FE, Cianci GC, Cipriani NA, et al. Lung cancer-a fractal viewpoint. Nat Rev Clin Oncol. 2015; 12:664–675. PMID: 26169924.
13. Liu S, Wang Y, Xu K, et al. Relationship between necrotic patterns in glioblastoma and patient survival: fractal dimension and lacunarity analyses using magnetic resonance imaging. Sci Rep. 2017; 7:8302. PMID: 28814802.
14. Liu S, Fan X, Zhang C, et al. MR imaging based fractal analysis for differentiating primary CNS lymphoma and glioblastoma. Eur Radiol. 2019; 29:1348–1354. PMID: 30167811.
15. Smitha KA, Gupta AK, Jayasree RS. Fractal analysis: fractal dimension and lacunarity from MR images for differentiating the grades of glioma. Phys Med Biol. 2015; 60:6937–6947. PMID: 26305773.
16. Falconer K. Fractal geometry: mathematical foundations and applications. Hoboken: John Wiley & Sons;2004.
17. Plotnick RE, Gardner RH, O'Neill RV. Lacunarity indices as measures of landscape texture. Landsc Ecol. 1993; 8:201–211.
18. Metze K. Fractal dimension of chromatin: potential molecular diagnostic applications for cancer prognosis. Expert Rev Mol Diagn. 2013; 13:719–735. PMID: 24063399.
19. Kane AJ, Sughrue ME, Rutkowski MJ, et al. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer. 2011; 117:1272–1278. PMID: 21381014.
20. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.
21. Sanghera B, Banerjee D, Khan A, et al. Reproducibility of 2D and 3D fractal analysis techniques for the assessment of spatial heterogeneity of regional blood flow in rectal cancer. Radiology. 2012; 263:865–873. PMID: 22438361.
22. Park YW, Kim S, Ahn SS, et al. Magnetic resonance imaging-based 3-dimensional fractal dimension and lacunarity analyses may predict the meningioma grade. Eur Radiol. 2020; 4. 09. DOI: 10.1007/s00330-020-06788-8. [Epub].
23. Nakasu S, Nakasu Y, Nakajima M, Matsuda M, Handa J. Preoperative identification of meningiomas that are highly likely to recur. J Neurosurg. 1999; 90:455–462. PMID: 10067913.
24. Siegers HP, Zuber P, Hamou MF, van Melle GD, de Tribolet N. The implications of the heterogeneous distribution of Ki-67 labelled cells in meningiomas. Br J Neurosurg. 1989; 3:101–107. PMID: 2789703.
25. Coroller TP, Bi WL, Huynh E, et al. Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS One. 2017; 12:e0187908. PMID: 29145421.
26. Park YW, Oh J, You SC, et al. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur Radiol. 2019; 29:4068–4076. PMID: 30443758.
27. Park YW, Han K, Ahn SS, et al. Whole-tumor histogram and texture analyses of DTI for evaluation of IDH1-mutation and 1p/19q-codeletion status in World Health Organization grade II gliomas. AJNR Am J Neuroradiol. 2018; 39:693–698. PMID: 29519794.
28. Park YW, Choi YS, Ahn SS, Chang JH, Kim SH, Lee SK. Radiomics MRI phenotyping with machine learning to predict the grade of lowergrade gliomas: a study focused on nonenhancing tumors. Korean J Radiol. 2019; 20:1381–1389. PMID: 31464116.
29. Park CJ, Choi YS, Park YW, et al. Diffusion tensor imaging radiomics in lower-grade glioma: improving subtyping of isocitrate dehydrogenase mutation status. Neuroradiology. 2020; 62:319–326. PMID: 31820065.
30. Park YW, Han K, Ahn SS, et al. Prediction of IDH1-mutation and 1p/19q-codeletion status using preoperative MR imaging phenotypes in lower grade gliomas. AJNR Am J Neuroradiol. 2018; 39:37–42. PMID: 29122763.
31. Park JE, Park SY, Kim HJ, Kim HS. Reproducibility and generalizability in radiomics modeling: possible strategies in radiologic and statistical perspectives. Korean J Radiol. 2019; 20:1124–1137. PMID: 31270976.
32. Toh CH, Castillo M, Wong AM, et al. Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am J Neuroradiol. 2008; 29:1630–1635. PMID: 18583409.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14791/btrt.2020.8.e3.
Fig. 1
The schematic of segmentation and fractal analysis in our study. A: A post-contrast T1-weighted imaging of a representative case with meningioma. B: After segementation of the enhancing portion of the tumor, two-dimensional and three-dimensional fractal analysis were performed by using box-counting methods.
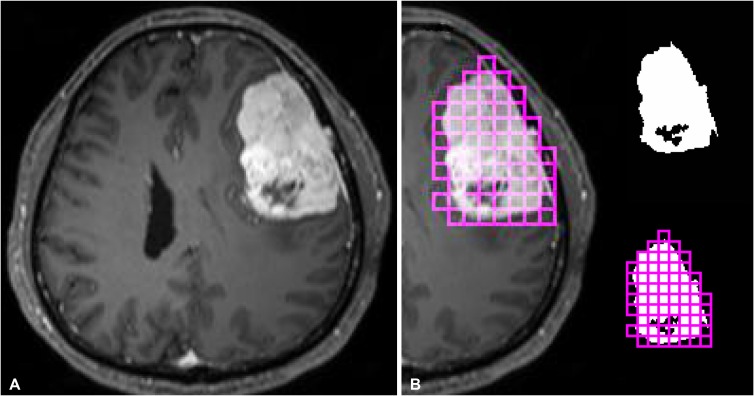
Fig. 2
Boxplot representation of the FD (A,C) and lacunarity (B, D) according to different meningioma grades. FD, fractal dimension; 2D, two-dimensional; 3D, three-dimensional.

Fig. 3
Receiver operating characteristic curves of 2D fractal models and 3D fractal models for predicting meningioma grades. 2D, two-dimensional; 3D, three-dimensional.
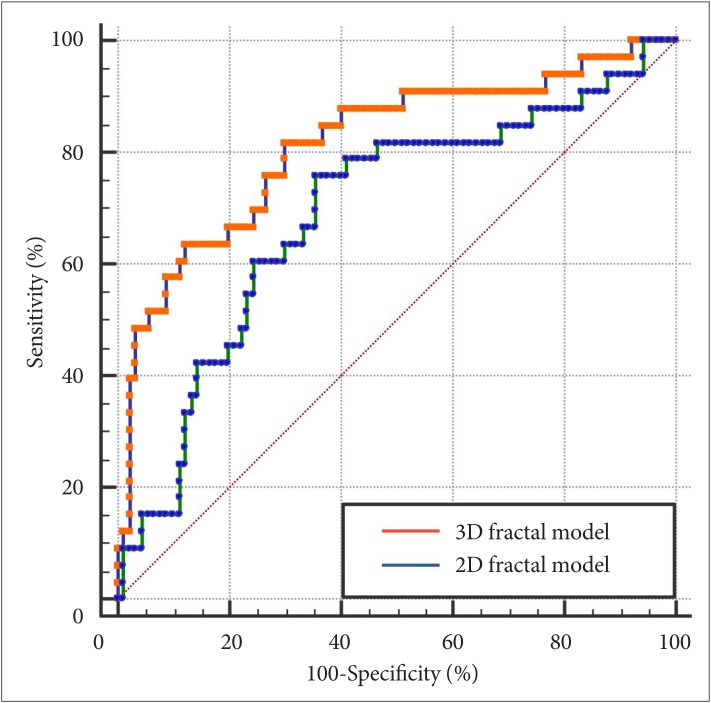
Table 1
Clinical and imaging characteristics according to the meningioma grade

Data are presented either numbers of patients (%) or mean±standard deviation. *Calculated from the Student t-test or Mann-Whitney U-test for continuous variables, and chi-square test for categorical variables. WHO, World Health Organization; FD, fractal dimension; 2D, two-dimensional; 3D, three-dimensional
Table 2
Univariate and multivariable logistic regression analysis for predictors of meningioma grade

Table 3
Comparison of diagnostic performance between 2D fractal model and 3D fractal model for predicting the meningioma grade

| Models | AUC (95% CI) | Accuracy (%) | Sensitivity (%) | Specificity (%) | p value* |
|---|---|---|---|---|---|
| 2D fractal model | 0.690 (0.581–0.799) | 72.4 | 75.8 | 64.4 | Reference |
| 3D fractal model | 0.813 (0.733–0.878) | 82.9 | 81.8 | 70.0 | <0.001 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download