Abstract
Background
Radiation therapy, one of the strongest anti-cancer treatments, is already performed to treat primary glioblastoma; however, the effect of repeated radiation therapy for recurrent tumors has not been fully explored. The aim of this study was to determine the efficacy of re-irradiation in treating recurrent glioblastoma.
Methods
The study included 36 patients with recurrent glioblastoma treated with repeated radiation therapy between 2002 and 2016. Stereotactic radiosurgery (SRS) and hypo-fractionated stereotactic radiotherapy (HSRT) were performed in these patients.
Results
Fourteen patients received SRS with a median dose of 25 Gy (range, 20–32 Gy) in 1–5 fractions. Twenty-two patients received HSRT with a median dose of 40 Gy (range, 31.5–52 Gy) in 6–20 fractions. There were six treatment-related grade 3 adverse events. Survival analysis showed that re-irradiation significantly prolonged overall survival (OS) and progression-free survival (PFS). The median OS and one-year OS rate after re-irradiation were 17.2 months and 60.4%, respectively. The median PFS and 6-month PFS rate after re-irradiation were 4.4 months and 41.9%, respectively. Of the 36 patients, three survived without any progression in their condition.
The standard treatment for newly diagnosed glioblastoma is maximum surgical resection followed by concurrent chemoradiotherapy (CCRT). Despite receiving standard treatment, most glioblastoma patients experience a recurrence within 1 year [12]. Several therapeutic options for recurrent glioblastoma have been investigated; however, there is no standard therapy that might significantly improve survival [34].
The therapeutic effect of re-irradiation for recurrent glioblastoma patients has been controversial [5]. Healthy brain tissue is vulnerable to damage to additional irradiation because of the initial radiation therapy. Recent advances in radiation techniques have made highly precise re-irradiation possible [6]. Stereotactic radiosurgery (SRS) and hypo-fractionated stereotactic radiotherapy (HSRT) can deliver high-dose radiation to tumors, while saving adjacent normal tissue. SRS and HSRT have been performed as salvage treatments and showed a favorable clinical outcome in patients with recurrent glioma [278].
The aim of this study was to determine the efficacy of re-irradiation in the treatment of recurrent glioblastoma.
The study included 36 patients with recurrent glioblastoma treated with re-irradiation therapy between January 2002 and December 2016. Postoperative positron emission tomography, MRI, and Karnofsky Performance Score (KPS) were considered important for deciding re-irradiation. SRS and HSRT were performed as re-irradiation therapies. Medical records that included demographic data and pathological, and radiological reports were reviewed retrospectively. All patients had pathologically confirmed glioblastoma. They progressed after standard CCRT with a median dose of 60 Gy (range, 54–66 Gy). Of the 36 patients, 31 (86.1%) received re-irradiation upon first recurrence with or without combined therapy. Re-irradiation was administered upon second recurrence in four patients and upon third recurrence in one patient. This retrospective study was approved by the Institutional Review Board of Bundang CHA Medical Center (2017-05-006-002).
SRS and HSRT were performed using Novalis (Varian Medical Systems, Inc., Palo Alto, CA, USA). T1 and T2 fluid-attenuated inversion recovery MRI were fused with simulation CT images with 1.5-mm slice thickness. Gross tumor volume (GTV) was defined as the contrast-enhancing tumor volume on T1-weighted MRI. The planning target volume (PTV) was the GTV plus a 3-mm isotropic margin. Peritumoral edema and critical structures were not included in the PTV. When the GTV diameter was greater than 3 cm, the PTV was not applied (Fig. 1).
Survival outcome and recurrence were estimated from the diagnosis and time of re-irradiation. Progression after re-irradiation was determined based on neuroimaging findings and symptoms. Postoperative tumor status was defined as gross total resection (GTR) if the postoperative MRI revealed no evidence of residual lesion. The extent of resection was defined as subtotal resection (STR) (<99%), near-total resection (NTR) (≥99%, <100%) based on postoperative MRI. Treatment-related toxicity was evaluated using the Common Terminology Criteria for Adverse Events version 4.03 (CTCAE v4.03) [9].
Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan–Meier method. Multivariate logistic regression analysis was used to assess the correlation between variables and survival. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 22, IBM Corp., Armonk, NY, USA).
Patient characteristics are summarized in Table 1. The median age was 50 years (range, 19–74 years). Twenty-four patients (66.7%) were men. Half of the patients had a KPS of ≥70. Only three patients underwent biopsy; 20 (60.6%) underwent GTR or NTR. The median initial tumor size was 7.5 cm (range, 3.0–12.7 cm). All patients had previously received concurrent temozolomide and radiotherapy. The genomic data were limited. O6-methylguanine-DNA methyltransferase (MGMT) methylation was positive in 19.4% of patients. There were no patients with isocitrate dehydrogenase-1 (IDH-1) mutations or 1p19q co-deletions. The median Ki-67 was 25% (range, 2.7–60.0%). Ten patients (27.8%) had Ki-67 ≥30%.
The median follow-up duration from initial diagnosis and reirradiation was 19.9 months (range, 7–177 months) (Table 2). The median recurrence time after CCRT was 7.1 months (range, 1–92.7 months). The locations of the first recurrences were as follows: in-field (25 patients; 69.4%), out-field (four patients; 11.1%), and in- and out-field (seven patients; 19.4%). The median size of the recurrent tumor was 5.5 cm (range, 1.4–13.8 cm). Surgical resection was performed in 15 patients upon first recurrence. A total of 17 patients (47.2%) underwent surgery; of these 17 patients, 11 (64.7%) underwent GTR or NTR. Genomic data from the salvage operation were also limited. No patient presented with MGMT methylation or IDH-1 mutations. The median Ki-67 was 20% (range, 10–60%). Re-irradiation was delivered as SRS/FSRS in 14 patients (38.9%) and HSRT in 22 patients (61.1%) (Table 3). The median total dose of re-irradiation was 32 Gy (range, 18–45 Gy), and the equivalent dose in 2 Gy fractions (EQD2) was 46.7 Gy (range, 31.3–68.0 Gy). The median fractional dose of SRS/FSRS was 8 Gy (range, 5–24 Gy) and that of HSRT was 4 Gy (range, 2.5– 5.5 Gy). The median PTV was 27 cm3 (range, 0.4–192.8 cm3).
After re-irradiation, three patients achieved complete remission, while 19 developed progressive disease. Of the 31 patients with available follow-up recurrence data, 22 experienced a second recurrence. Nineteen patients received additional treatment; 14 received re-irradiation after the second recurrence. At the final follow-up, 14 patients had subsequently died, while three showed no evidence of disease.
The median OS and one-year OS rate after initial diagnosis were 38 months and 91.3%, respectively, and the median survival and one-year OS rates after re-irradiation were 17.2 months and 60.4%, respectively. The median progression time and 6-month PFS rates after re-irradiation were 4.4 months and 41.9%, respectively (Fig. 2).
In the univariate analysis, recurrence location (in-field only) and surgical resection were prognostic factors for superior OS (p=0.013 and 0.010, respectively) (Table 4). Analysis of salvage chemotherapy, re-irradiation methods, re-irradiation dose, and interval between CCRT and first recurrence showed that the recurrent tumor size bore no relation to survival rate. Salvage radiotherapy at first recurrence seemed to yield superior OS (p=0.063) rates. In the multivariate analysis, surgical resection was a significant prognostic factor for superior OS (p=0.015) rates (Fig. 3). We could not find a significant prognostic factor for PFS.
Acute toxicity occurred in 22 patients (61.1%); most of these were grade 1 (18.2%) and 2 (63.6%). There were six treatment-related grade 3 adverse events. Radiation necrosis with edema occurred in four patients in the acute period and in two in the late period. Patients with grade 1 and 2 toxicity were given conservative medical treatment including steroids. Three patients in the acute phase and one patient in the late phase with grade 3 hydrocephalus underwent ventriculoperitoneal shunt procedures.
We investigated the effects of re-irradiation using SRS/HSRT for recurrent glioblastoma; both methods had favorable outcomes, with a median PFS of 4.4 months and OS of 17.2 months after re-irradiation. Re-irradiation for recurrent glioblastoma showed good survival outcomes. There was no grade 4 or 5 toxicity. The median PFS and OS after the first recurrence were 16.5 and 19.5 months, respectively.
Our results are in accordance with those of previous studies [781011121314151617]. The OS ranged between 8 and 12.4 months. In addition, tumor volume is a poor prognostic factor. Although tumor volumes in the present study were larger (27 cm3) than those recorded in previous studies, we reported a median OS of 17.2 months.
In this study, patients treated with re-irradiation and surgical resection showed superior OS. Surgical resection of recurrent glioblastoma has a favorable outcome [1819]. In addition, surgical resection may allow re-irradiation with reduced cumulative normal brain doses. Even though radiotherapy techniques have advanced, re-irradiation still confers a risk of radiation injury. Surgical resection before re-irradiation could decrease tumor volumes, resulting in decreased re-irradiation target volumes. Decreased target volumes could reduce radiation doses to adjacent normal brain tissue. Martínez-Carrillo et al. [20] reported that 59% of patients could receive SRS due to a reduction in tumor volume through surgical resection.
In most cases, progression of glioblastoma occurred in field of the initial radiotherapy. Because of previous high doses of radiation, physicians worry about the cumulative effect of radiation and damage to healthy brain tissue damage. There is no standardized re-irradiation scheme and re-irradiation doses vary across the literature. We could not find any significant prognostic factors in the re-irradiation scheme or doses. There was no dose-response (above or below EQD2 50 Gy). The method of re-irradiation (SRS/FSRS or HSRT) and tumor volume did not show any differences in survival rates.
At the last follow-up, three of the 36 patients had survived with no progression in their condition. All three patients had in-field recurrence only and a KPS of ≥70. The recurrence time after CCRT was over one year in two patients, both of whom had undergone surgical resection. The recurrent tumors were less than 5 cm in size. None of the patients presented with favorable molecular features. One patient who received carmustine chemotherapy after re-irradiation was confirmed to have radiologic progression on follow-up MRI.
Four patients in the acute period and two in the late period had grade 3 treatment-related toxicity. Patients with grade 3 headaches (one patient in the acute period and one patient in the late period) were admitted and received conservative care. Four patients (three patients in the acute period and one patient in the late period) underwent ventriculoperitoneal shunt procedures for hydrocephalus. There was no grade 4 or 5 toxicity. Re-irradiation using SRS and HSRT resulted in less treatment-related toxicity than brachytherapy. Brachytherapy is an invasive procedure that confers a risk of infection and hemorrhage. A study of salvage brachytherapy for recurrent glioma reported 6% grade 3, 1% grade 4, and 1% grade 5 toxicity [21].
This study has some limitations. Because of its retrospective design, we had a small heterogeneous population and heterogeneous re-irradiation scheme. Re-irradiation with combined treatment was performed non-uniformly. We could not investigate the effect of combined treatments, such as chemotherapy or hyperthermia. Additionally, it was difficult to determine whether the toxicity was related to re-irradiation, other combined treatments, and/or disease progression; therefore, treatment-related toxicity might have been underreported.
In conclusion, re-irradiation for recurrent glioblastoma showed favorable outcomes. Surgical resection should be given careful consideration due to the decreased re-irradiation target volumes.
Acknowledgments
This research was supported by grants of Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Korea Government (Grant No. 2018R1C1B5086460).
References
1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996. PMID: 15758009.
2. Patel M, Siddiqui F, Jin JY, et al. Salvage reirradiation for recurrent glioblastoma with radiosurgery: radiographic response and improved survival. J Neurooncol. 2009; 92:185–191. PMID: 19066727.
3. Gallego O. Nonsurgical treatment of recurrent glioblastoma. Curr Oncol. 2015; 22:e273–e281. PMID: 26300678.
4. Roy S, Lahiri D, Maji T, Biswas J. Recurrent glioblastoma: where we stand. South Asian J Cancer. 2015; 4:163–173. PMID: 26981507.
5. Redmond KJ, Mehta M. Stereotactic radiosurgery for glioblastoma. Cureus. 2015; 7:e413. PMID: 26848407.
6. Ryu S, Buatti JM, Morris A, et al. The role of radiotherapy in the management of progressive glioblastoma : a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014; 118:489–499. PMID: 24728785.
7. Torok JA, Wegner RE, Mintz AH, Heron DE, Burton SA. Re-irradiation with radiosurgery for recurrent glioblastoma multiforme. Technol Cancer Res Treat. 2011; 10:253–258. PMID: 21517131.
8. Holt DE, Bernard ME, Quan K, et al. Salvage stereotactic radiosurgery for recurrent glioblastoma multiforme with prior radiation therapy. J Cancer Res Ther. 2016; 12:1243–1248. PMID: 28169234.
9. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.3. Bethesda, MD: U.S. Department of Health and Human Services;2010. Accessed April 19, 2017. at https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
10. Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010; 28:3048–3053. PMID: 20479391.
11. Maranzano E, Anselmo P, Casale M, et al. Treatment of recurrent glioblastoma with stereotactic radiotherapy: long-term results of a mono-institutional trial. Tumori. 2011; 97:56–61. PMID: 21528665.
12. Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012; 82:2018–2024. PMID: 21489708.
13. Minniti G, Scaringi C, De Sanctis V, et al. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol. 2013; 111:187–194. PMID: 23129347.
14. Greenspoon JN, Sharieff W, Hirte H, et al. Fractionated stereotactic radiosurgery with concurrent temozolomide chemotherapy for locally recurrent glioblastoma multiforme: a prospective cohort study. Onco Targets Ther. 2014; 7:485–490. PMID: 24711705.
15. Minniti G, Agolli L, Falco T, et al. Hypofractionated stereotactic radiotherapy in combination with bevacizumab or fotemustine for patients with progressive malignant gliomas. J Neurooncol. 2015; 122:559–566. PMID: 25702193.
16. Kim BS, Kong DS, Seol HJ, Nam DH, Lee JI. MGMT promoter methylation status as a prognostic factor for the outcome of gamma knife radiosurgery for recurrent glioblastoma. J Neurooncol. 2017; 133:615–622. PMID: 28536992.
17. Back M, Gzell CE, Kastelan M, Guo L, Wheeler HR. Large volume re-irradiation with bevacizumab is a feasible salvage option for patients with refractory high-grade glioma. Neurooncol Pract. 2015; 2:48–53. PMID: 26034641.
18. Azoulay M, Santos F, Shenouda G, et al. Benefit of re-operation and salvage therapies for recurrent glioblastoma multiforme: results from a single institution. J Neurooncol. 2017; 132:419–426. PMID: 28374095.
19. Lu VM, Goyal A, Graffeo CS, et al. Survival benefit of maximal resection for glioblastoma reoperation in the temozolomide era: a meta-analysis. World Neurosurg. 2019; 127:31–37. PMID: 30947000.
20. Martínez-Carrillo M, Tovar-Martín I, Zurita-Herrera M, et al. Salvage radiosurgery for selected patients with recurrent malignant gliomas. Biomed Res Int. 2014; 2014:657953. PMID: 24895599.
21. Scharfen CO, Sneed PK, Wara WM, et al. High activity iodine-125 interstitial implant for gliomas. Int J Radiat Oncol Biol Phys. 1992; 24:583–591. PMID: 1429079.
Fig. 1
Treatment case of re-irradiation therapy. Recurrent tumors were diagnosed in a 56-year-old woman 15 months after the initial operation. The patient underwent an operation and re-irradiation of 24-Gy in four fractions. A: Magnetic resonance imaging (MRI) at initial diagnosis. Left: T1 enhanced MRI, Right: T2 fluid-attenuated inversion recovery MRI. B: MRI after the first operation. C: MRI at recurrence. D: MRI after the second operation. E: Re-irradiation treatment plan. Red: planning target volume; orange: 95% iso-dose line; yellow: 90% iso-dose line; light green: 80% iso-dose line; sky blue: 40% iso-dose line. F: MRI 7 months after re-irradiation
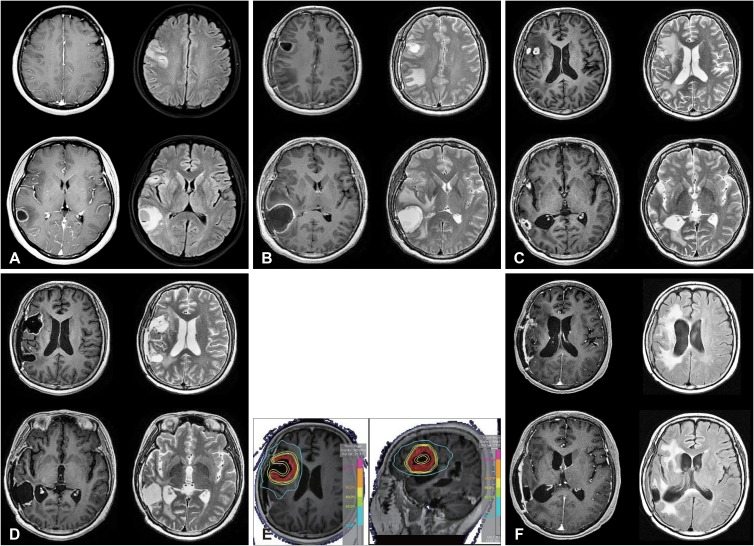
Table 1
Patient characteristics
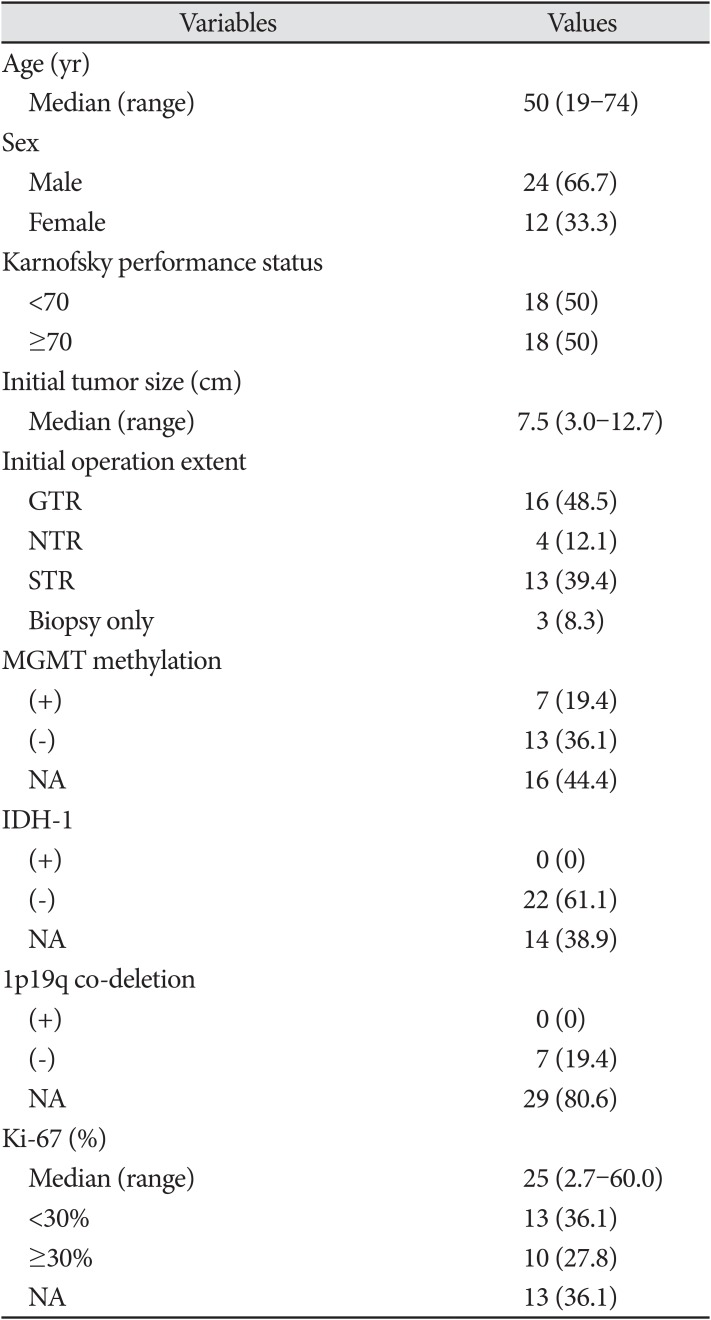
Table 2
Recurrence and treatment
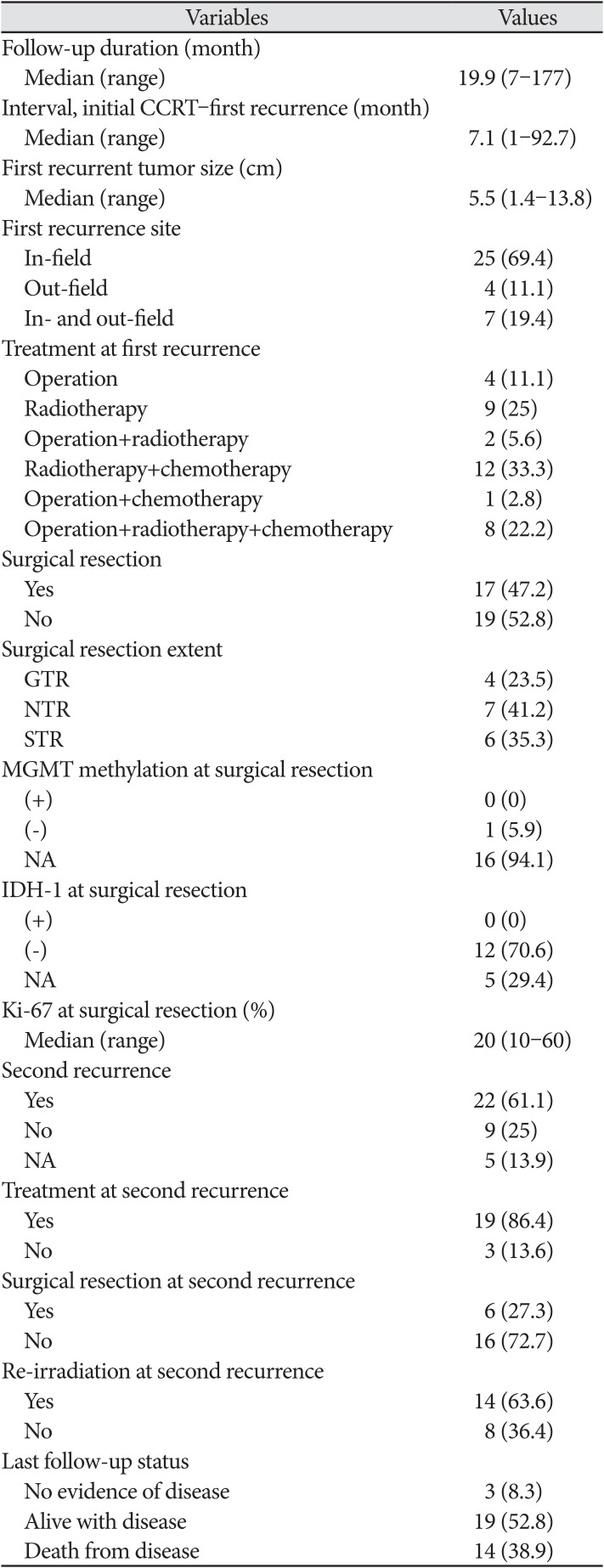
Table 3
Re-irradiation scheme
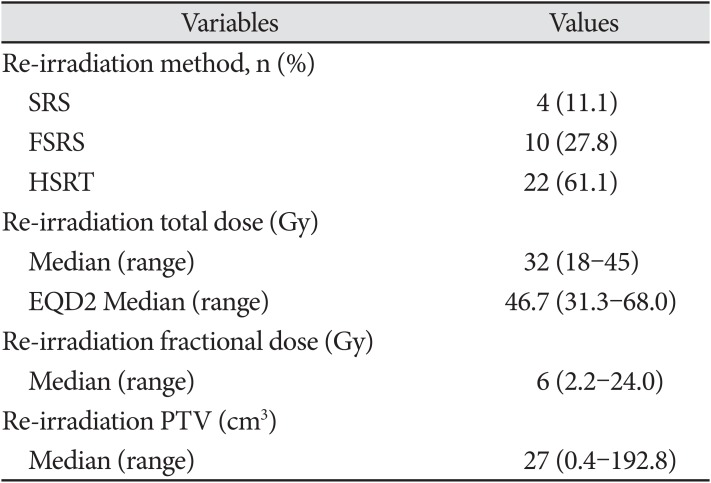
Table 4
Univariate analysis for survival
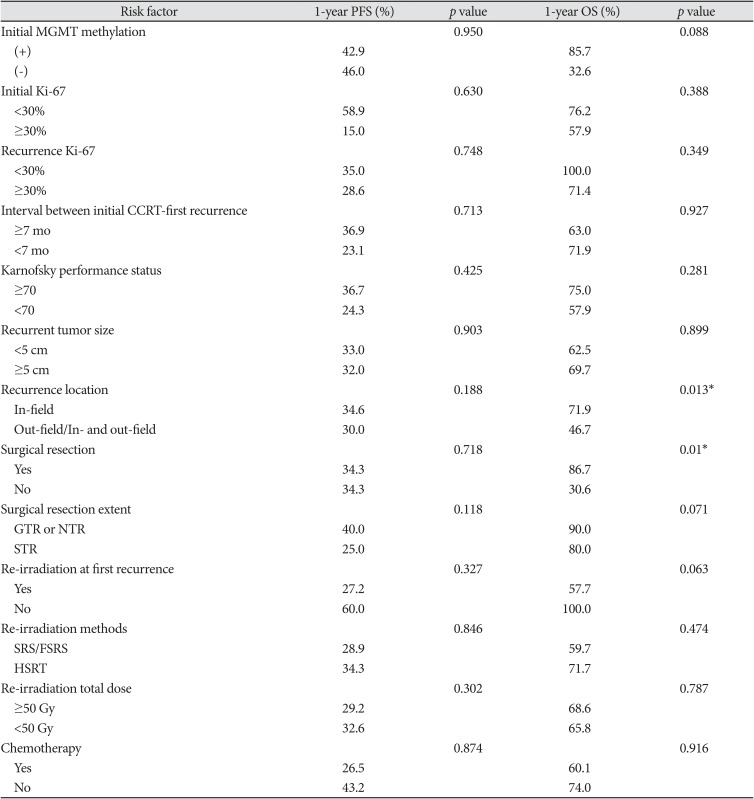
*p<0.05. PFS, progression-free survival; OS, overall survival; MGMT, O6-methylguanine-DNA methyltransferase; CCRT, concurrent chemoradiotherapy; GTR, gross total resection; NTR, near-total resection; STR, subtotal resection; SRS, stereotactic radiosurgery; FSRS, fractionated stereotactic radiosurgery; HSRT, hypo-fractionated stereotactic radiotherapy




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download