Abstract
From 2004 to 2020, we studied three pediatric patients (age: 9–13 years, all male) and one adult patient (age: 29 years, female) with tectal plate glioma with obstructing hydrocephalus on MRI. One patient had neurofibromatosis type 1. All patients complained about headaches and vomiting, and one patient had diplopia. Endoscopic third ventriculostomy (ETV) was underwent in all patients and a biopsy was obtained from two patients. Pathologic diagnoses were a pilocytic astrocytoma and a low-grade glioma. After ETV with or without biopsy, neurological symptoms were improved in all patients. Three patients did the clinical and radiological follow-up without adjuvant treatment. One patient underwent gamma knife radiosurgery. In two pediatric patients and the adult patient, there was no clinical and radiological progression after 6.2, 6.9, and 8.0 years, respectively. One pediatric patient whose lesion had focal enhancement had radiologic progression without any neurologic symptoms after 5.1 years. Without adjuvant treatment for this lesion, there was no clinical deterioration neither further radiological progression for 6.2 years after radiological aggravation. Tectal plate gliomas showed indolent clinical courses, even after radiologic tumor progression. After the treatment of obstructing hydrocephalus, clinical and radiologic follow-up can be recommended for indolent tectal plate gliomas.
Brain stem gliomas in childhood account for 20% or more of primary brain tumors. In adults, they account for less than 2% of intracranial tumors [12]. Brain stem gliomas can be divided into diffuse intrinsic pontine gliomas, tectal plate gliomas, focal brainstem tumors, dorsal exophytic tumors, and cervicomedullary tumors, depending on the location. The treatment methods differ according to clinical symptoms, topographic localization, and imaging characteristics [3].
Unlike diffuse intrinsic pontine gliomas, which account for 80% of brainstem gliomas, tectal plate gliomas tend to progress slowly and are clinically indolent with fewer neurologic symptoms except increased intracranial pressure symptoms due to obstructive hydrocephalus [4]. Since obstructive hydrocephalus can be treated via diversion of the cerebrospinal fluid, follow-up imaging and wait-and-see approach may be the treatment of choice for indolent tectal plate gliomas without radiation therapy or chemotherapy. In this study, we retrospectively reviewed the clinical course of four patients with tectal plate gliomas in our institution.
This retrospective study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (CNUHH-2020-138). This manuscript does not contain any individual's data in any form and the need for written informed was waived. The patient's information was summarized in Table 1. From 2004 to 2020, there were three pediatric patients (age: 9–13 years, all male) and one adult patient (age: 29 years, female) with tectal plate gliomas with obstructing hydrocephalus on MRI. They had been followed for more than five years. Brain MRI showed the tectal plate lesions with hypointensity on T1-weighted images and hyperintensity on T2-weighted images. After gadolinium administration, there was no enhancement except one patient. One patient had neurofibromatosis type 1. All patients complained about headaches and vomiting, and one patient had diplopia. Endoscopic third ventriculostomy (ETV) was conducted in all patients and biopsies were obtained from two patients. Pathologic diagnoses were a pilocytic astrocytoma and a low-grade glioma.
After ETV with or without biopsy, neurological symptoms were improved in all patients. Three patients underwent the clinical and radiological follow-up without adjuvant treatment, and one patient underwent gamma knife radiosurgery with 8 Gy at 50% isodose curve for 2.4 cm3. In two pediatric patients and the adult patient, there was no clinical and radiological progression after 6.2, 6.9, and 8.0 years. Brain MRI showed no change in the tectal plate lesion without hydrocephalus. One pediatric patient whose lesion had focal enhancement displayed a radiologic progression without any neurological symptoms after 5.1 years. Without adjuvant treatment for this lesion, there was no clinical deterioration neither further radiological progression for 6.2 years after radiological aggravation.
A 9-year-old boy complaint with headache and diplopia. The brain MRI revealed hydrocephalus and a 1.3-cm-sized tectal plate glioma with focal enhancement (Fig. 1A, B). He underwent ETV upon which his neurological symptoms improved. Postoperative follow-up brain MRI showed tectal plate glioma without hydrocephalus (Fig. 1C).
On a regular follow-up 5.1 years after the ETV, the size of the tectal plate glioma was increased without contrast media enhancement (Fig. 1D, E). On C-methionine brain PET/CT images, the hypermetabolic lesion was detected (Fig. 1F). The patient did not show any neurological symptoms. As his family did not want further treatment such as chemotherapy and radiotherapy, he underwent close clinical and radiological follow-up. Without adjuvant treatment for this lesion, there was no clinical deterioration neither further radiological progression for 6.2 years after radiological aggravation.
Brainstem gliomas differ in their progression rates depending on the histology of the tumor, the location within the brainstem, clinical symptoms, and the response to therapy [12]. Brainstem gliomas without pontine involvement are almost always low-grade tumors and have an excellent outcome [5]. Tectal plate gliomas are a distinct subgroup of brainstem gliomas with indolent clinical progress [4]. They are usually diagnosed in childhood and do often manifest in adults. The mean age at diagnosis is 7–10 years. Brain MR images typically reveal an intrinsic focal lesion of the tectum without contrast enhancement [6]. Tectal plate lesions are located close to the cerebral aqueduct and cause hydrocephalus by slowly plugging the aqueduct of the Sylvius. Indolent tectal plate gliomas characteristically cause few other neurological symptoms. Most patients present symptoms of increased intracranial pressures related to obstructing hydrocephalus. Their clinical symptoms are headaches, papilledema, and vomiting. Clinical symptoms can be managed with cerebrospinal fluid (CSF) diversion procedures. Mostly, CSF diversion such as ETV or ventriculoperitoneal shunts and yearly radiologic follow-up may be sufficient for treating tectal plate gliomas. However, in rare cases, they display invasive clinical behavior [57].
Radiologically, tectal plate gliomas typically show iso-signal intensity on T1-weighted MR images and high-signal intensity on T2-weighted images localized to the midbrain tectum [468]. Gadolinium enhancement is not observed. The tumor is usually smaller than 2 cm in diameter. Most tectal plate gliomas tend to remain stable without imaging progression. In the rare cases of tumor progression, a typical tectal plate glioma is seen as a globular enlargement with a well-demarcated intrinsic lesion with iso-hypointensity on T1-weighted images and high intensity on T2-weighted images without contrast enhancement [68]. Larger tumor size and contrast enhancement are factors that can predict tumor progression and are the most critical radiological parameters for determining further treatment [9]. If the tumor is larger than 2 cm in diameter, is a contrast-enhancing lesion, and invades nearby structures including the pons, it may indicate a malignant tumor [51011]. One study demonstrated a highly significant correlation between tumor location and malignant behavior [5]. Depending on the location, 91% of tumors involving only the pons are malignant compared to only 2% of the non-pontine lesions. Tumors with partial pontine involvement are malignant in 14–37% of the cases [5].
Most intrinsic tectal plate gliomas are low-grade tumors [5911]. World Health Organization grade I or grade II astrocytoma is the most common lesion encountered. In this study, two out of four patients pathologically showed a pilocytic astrocytoma and a low-grade glioma. The major limitation of this study is that not all have been confirmed pathologically and there was no genetic information. While complete resection is the treatment of choice for low-grade glioma in other brain locations, brainstem low-grade gliomas are associated with a risk of significant morbidity caused by surgery. Due to the high morbidity rate, a cautious approach for tumor biopsy or limited debulking should be considered. Children with brainstem low-grade tumors have an excellent outcome with initial radiological observation and there was no clear benefit for radiotherapy over chemotherapy when adjuvant treatment was needed [5]. Considering the clinical course, the first-line treatment consists of CSF diversion without biopsy and serial radiological follow-up. Surgery in addition to CSF diversion, radiotherapy, or chemotherapy may be required if the tumor progresses. Radiologic tumor progression occurs in 15–25% of cases [12]. However, even if radiological progression is detected, not many of these cases, in particular those with tectal plate gliomas, show clinical progression [13]. No clinical progression was noted for 1.8–6.9 years after radiological progression. The additional treatment could be reserved for patients with both radiological and clinical progression of tectal plate gliomas. Surgical procedures followed by radiotherapy with or without chemotherapy may be recommended for more aggressive tectal plate gliomas after a radiologic follow-up [457].
In summary, tectal plate gliomas showed indolent clinical courses, even after radiologic tumor progression. Most tectal plate gliomas do not cause any symptoms other than increased intracranial pressure due to obstructing hydrocephalus. After CSF diversion, a clinical and radiologic follow-up is sufficient in the absence of other neurological symptoms.
References
1. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012; 14 Suppl 5:v1–v49. PMID: 23095881.
2. Grimm SA, Chamberlain MC. Brainstem glioma: a review. Curr Neurol Neurosci Rep. 2013; 13:346. PMID: 23512689.
3. Smith MA, Freidlin B, Ries LA, Simon R. Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1998; 90:1269–1277. PMID: 9731733.
4. Dağlioğlu E, Cataltepe O, Akalan N. Tectal gliomas in children: the implications for natural history and management strategy. Pediatr Neurosurg. 2003; 38:223–231. PMID: 12686764.
5. Fried I, Hawkins C, Scheinemann K, et al. Favorable outcome with conservative treatment for children with low grade brainstem tumors. Pediatr Blood Cancer. 2012; 58:556–560. PMID: 21618421.
6. Bognar L, Turjman F, Villanyi E, et al. Tectal plate gliomas. Part II: CT scans and MR imaging of tectal gliomas. Acta Neurochir (Wien). 1994; 127:48–54. PMID: 7942181.
7. Matsuno A, Nagashima H, Ishii H, Iwamuro H, Nagashima T. Aggressive and invasive growth of tectal glioma after surgical intervention and chemoradiotherapy. Br J Neurosurg. 2006; 20:246–249. PMID: 16954079.
8. May PL, Blaser SI, Hoffman HJ, Humphreys RP, Harwood-Nash DC. Benign intrinsic tectal "tumors" in children. J Neurosurg. 1991; 74:867–871. PMID: 2033445.
9. Squires LA, Allen JC, Abbott R, Epstein FJ. Focal tectal tumors: management and prognosis. Neurology. 1994; 44:953–956. PMID: 8190303.
10. Wang C, Zhang J, Liu A, Sun B, Zhao Y. Surgical treatment of primary midbrain gliomas. Surg Neurol. 2000; 53:41–51. PMID: 10697232.
11. Pollack IF, Pang D, Albright AL. The long-term outcome in children with late-onset aqueductal stenosis resulting from benign intrinsic tectal tumors. J Neurosurg. 1994; 80:681–688. PMID: 8151347.
12. Stark AM, Fritsch MJ, Claviez A, Dörner L, Mehdorn HM. Management of tectal glioma in childhood. Pediatr Neurol. 2005; 33:33–38. PMID: 15876519.
13. Bowers DC, Georgiades C, Aronson LJ, et al. Tectal gliomas: natural history of an indolent lesion in pediatric patients. Pediatr Neurosurg. 2000; 32:24–29. PMID: 10765135.
Fig. 1
Case 3: Tectal plate glioma with radiological progression but without clinical deterioration. A: Brain MRI revealed a tectal plate lesion with hyperintensity on T2-weighted images. B: After gadolinium administration, the lesion showed a focal enhancement. C: Postoperative follow-up brain MRI revealed a tectal plate glioma without hydrocephalus. D, E: In a follow-up 5.1 years after endoscopic third ventriculostomy, the brain MRI showed that the size of the tectal plate glioma was increased without contrast media enhancement. F: On C-methionine brain PET/CT images, a hypermetabolic lesion was detected.
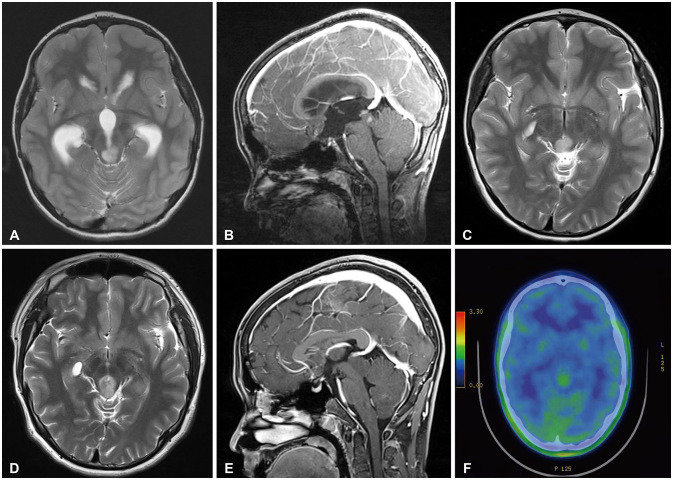
Table 1
The clinical characteristics of 4 tectal plate gliomas





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download