1. Santarelli JG, Sarkissian V, Hou LC, Veeravagu A, Tse V. Molecular events of brain metastasis. Neurosurg Focus. 2007; 22:E1.
2. Nathoo N, Chahlavi A, Barnett GH, Toms SA. Pathobiology of brain metastases. J Clin Pathol. 2005; 58:237–242. PMID:
15735152.
3. Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases—translation to new therapies. Nat Rev Clin Oncol. 2011; 8:344–356. PMID:
21487419.
4. Cox JD, Scott CB, Byhardt RW, et al. Addition of chemotherapy to radiation therapy alters failure patterns by cell type within non-small cell carcinoma of lung (NSCCL): analysis of Radiation Therapy Oncology Group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1999; 43:505–509. PMID:
10078629.
5. Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005; 23:6207–6219. PMID:
16135488.
6. Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003; 21:2529–2536. PMID:
12829672.
7. Paz-Ares L, Soulières D, Melezínek I, et al. Clinical outcomes in non-small-cell lung cancer patients with EGFR mutations: pooled analysis. J Cell Mol Med. 2010; 14:51–69. PMID:
20015198.
8. Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010; 12:1193–1199. PMID:
20627894.
9. Proto C, Imbimbo M, Gallucci R, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of central nervous system metastases from non-small cell lung cancer: the present and the future. Transl Lung Cancer Res. 2016; 5:563–578. PMID:
28149752.
10. Zhang J, Yu J, Sun X, Meng X. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of central nerve system metastases from non-small cell lung cancer. Cancer Lett. 2014; 351:6–12. PMID:
24861428.
11. Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptormutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 2012; 18:938–944. PMID:
22167408.
12. Soon YY, Leong CN, Koh WY, Tham IW. EGFR tyrosine kinase inhibitors versus cranial radiation therapy for EGFR mutant non-small cell lung cancer with brain metastases: a systematic review and meta-analysis. Radiother Oncol. 2015; 114:167–172. PMID:
25583566.
13. Qu J, Wang YN, Xu P, et al. Clinical efficacy of icotinib in lung cancer patients with different EGFR mutation status: a meta-analysis. Oncotarget. 2017; 8:33961–33971. PMID:
28430623.
14. Zhao B, Zhang W, Yu D, Xu J, Wei Y. Erlotinib in combination with bevacizumab has potential benefit in non-small cell lung cancer: a systematic review and meta-analysis of randomized clinical trials. Lung Cancer. 2018; 122:10–21. PMID:
30032815.
15. Buonerba C, Iaccarino S, Dolce P, et al. Predictors of outcomes in patients with EGFR-mutated non-small cell lung cancer receiving EGFR tyrosine kinase inhibitors: a systematic review and meta-analysis. Cancers (Basel). 2019; 11:1259.
16. Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010; 16:5873–5882. PMID:
21030498.
17. Heimberger AB, Learn CA, Archer GE, et al. Brain tumors in mice are susceptible to blockade of epidermal growth factor receptor (EGFR) with the oral, specific, EGFR-tyrosine kinase inhibitor ZD1839 (iressa). Clin Cancer Res. 2002; 8:3496–3502. PMID:
12429640.
18. Fekrazad MH, Ravindranathan M, Jones DV Jr. Response of intracranial metastases to erlotinib therapy. J Clin Oncol. 2007; 25:5024–5026. PMID:
17971603.
19. Popat S, Hughes S, Papadopoulos P, et al. Recurrent responses to non-small cell lung cancer brain metastases with erlotinib. Lung Cancer. 2007; 56:135–137. PMID:
17157952.
20. Gounant V, Wislez M, Poulot V, et al. Subsequent brain metastasis responses to epidermal growth factor receptor tyrosine kinase inhibitors in a patient with non-small-cell lung cancer. Lung Cancer. 2007; 58:425–428. PMID:
17945377.
21. Aiko N, Shimokawa T, Miyazaki K, et al. Comparison of the efficacies of first-generation epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in patients with advanced non-small-cell lung cancer harboring EGFR mutations. BMC Cancer. 2018; 18:1012. PMID:
30348116.
22. Dempke WC, Edvardsen K, Lu S, Reinmuth N, Reck M, Inoue A. Brain metastases in NSCLC - are TKIs changing the treatment strategy? Anticancer Res. 2015; 35:5797–5806. PMID:
26504000.
23. Gerber NK, Yamada Y, Rimner A, et al. Erlotinib versus radiation therapy for brain metastases in patients with EGFR-mutant lung adenocarcinoma. Int J Radiat Oncol Biol Phys. 2014; 89:322–329. PMID:
24679729.
24. Burel-Vandenbos F, Ambrosetti D, Coutts M, Pedeutour F. EGFR mutation status in brain metastases of non-small cell lung carcinoma. J Neurooncol. 2013; 111:1–10. PMID:
23086434.
25. Byeon S, Ham JS, Sun JM, et al. Analysis of the benefit of sequential cranial radiotherapy in patients with EGFR mutant non-small cell lung cancer and brain metastasis. Med Oncol. 2016; 33:97. PMID:
27447711.
26. Economopoulou P, Mountzios G. Non-small cell lung cancer (NSCLC) and central nervous system (CNS) metastases: role of tyrosine kinase inhibitors (TKIs) and evidence in favor or against their use with concurrent cranial radiotherapy. Transl Lung Cancer Res. 2016; 5:588–598. PMID:
28149754.
27. Shin SM, Cooper BT, Chachoua A, et al. Survival but not brain metastasis response relates to lung cancer mutation status after radiosurgery. J Neurooncol. 2016; 126:483–491. PMID:
26520640.
28. Cho KR, Lee MH, Kong DS, et al. Outcome of gamma knife radiosurgery for metastatic brain tumors derived from non-small cell lung cancer. J Neurooncol. 2015; 125:331–338. PMID:
26373297.
29. Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. 2010; 362:1119–1127. PMID:
20335588.
30. Miller JA, Kotecha R, Ahluwalia MS, et al. The impact of tumor biology on survival and response to radiation therapy among patients with non-small cell lung cancer brain metastases. Pract Radiat Oncol. 2017; 7:e263–e273. PMID:
28254368.
31. Kim HJ, Kim WS, Kwon DH, Cho YH, Choi CM. Effects of an epithelial growth factor receptor-tyrosine kinase inhibitor add-on in stereotactic radiosurgery for brain metastases originating from non-small-cell lung cancer. J Korean Neurosurg Soc. 2015; 58:205–210. PMID:
26539262.
32. Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011; 37:624–631. PMID:
20595147.
33. Na YC, Jung HH, Kim HR, et al. Predictive factors of early distant brain failure after gamma knife radiosurgery alone in patients with brain metastases of non-small-cell lung cancer. J Neurooncol. 2017; 132:333–340. PMID:
28074321.
34. Bragstad S, Flatebø M, Natvig GK, et al. Predictors of quality of life and survival following Gamma Knife surgery for lung cancer brain metastases: a prospective study. J Neurosurg. 2018; 129:71–83. PMID:
28820304.
35. Tini P, Nardone V, Pastina P, et al. Perilesional edema in brain metastasis from non-small cell lung cancer (NSCLC) as predictor of response to radiosurgery (SRS). Neurol Sci. 2017; 38:975–982. PMID:
28260188.
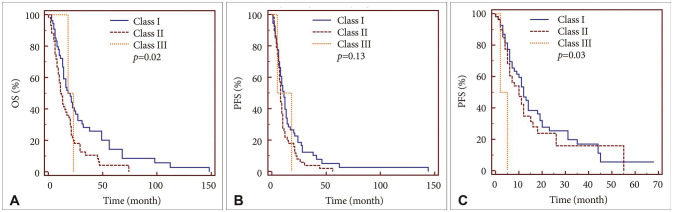
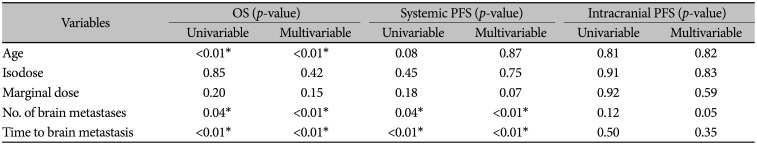




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download