Abstract
Background
Methods
Results
Appendix
Appendix
AED usage
( ) Presence of seizure in the peri-/post-operative period
( ) Usage of AED combination therapy
( ) Abnormality of EEG
( ) Location of brain tumor
( ) Type of brain tumor
( ) Presence of residual tumor
( ) Extent of peri-tumoral edema
( ) Extent of post-operative hemorrhages in surgical field (or intraoperative bleeding)
( ) Never (no permission of driving a car)
( ) Can drive a car immediately after quitting AEDs
( ) Can drive a car when seizure-free for several periods (1 month–2 years) after quitting AEDs
( ) Can drive a car when seizure-free at the time of follow-up regardless of taking AEDs
( ) Can drive a car when seizure-free at least 6 months regardless of taking AEDs
( ) Can drive a car when seizure-free at least 1 years regardless of taking AEDs
( ) Can drive a car when seizure-free at least 2 years regardless of taking AEDs
( ) Always (no restriction of driving a car)
Steroid usage
( ) Presence of underlying diseases (e.g. hypertension, diabetes, osteoporosis and immunosuppressive state) of patients
( ) Type of brain tumor (e.g. extraaxial or intraaxial tumor)
( ) Location and size of brain tumor
( ) Presence of peri-tumoral edema
( ) Degree of clinical symptoms
( ) Type of surgery (e.g. fluorescence guided surgery or awake surgery)
( ) Other
References
Fig. 1
Frequency of prescribing prophylactic antiepileptic drugs in brain tumor patients before and after surgery.
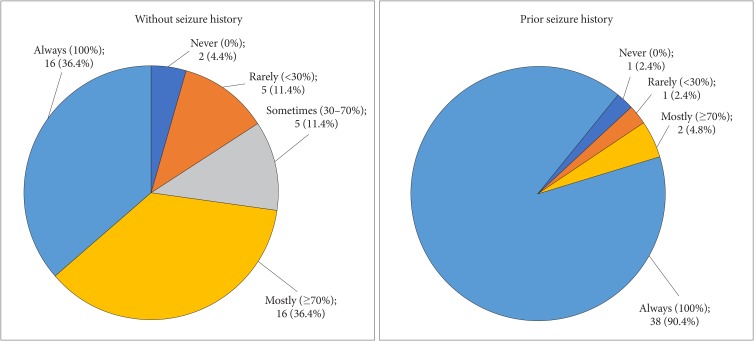
Fig. 2
Determination of duration of antiepileptic drug prophylaxis according to the seizure history before and after surgery.
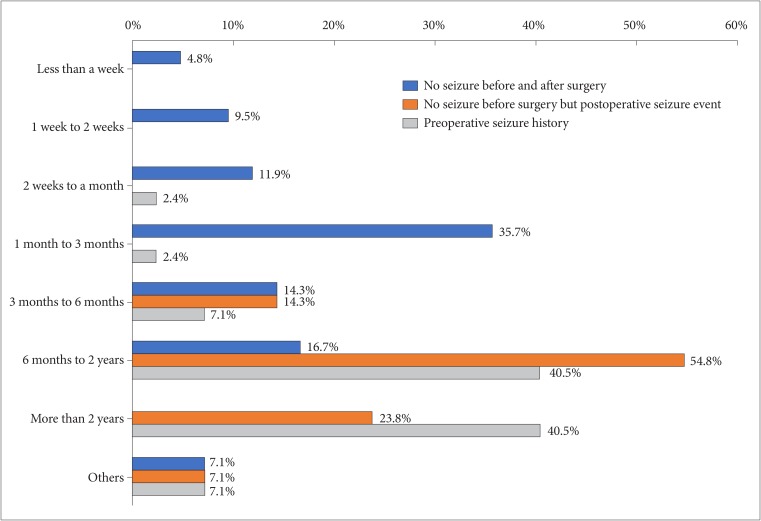
Fig. 3
Pie chart demonstrating widespread variability in the question of conditions allowing driving a car to patient with seizure.
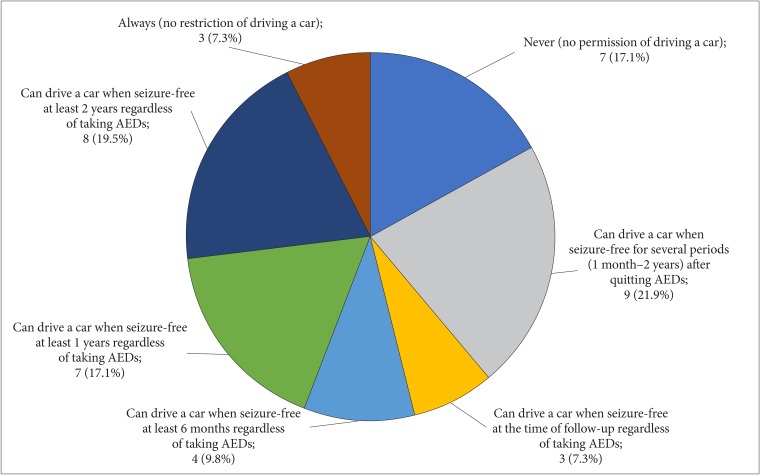
Table 1
Summary of the characteristics of the respondents
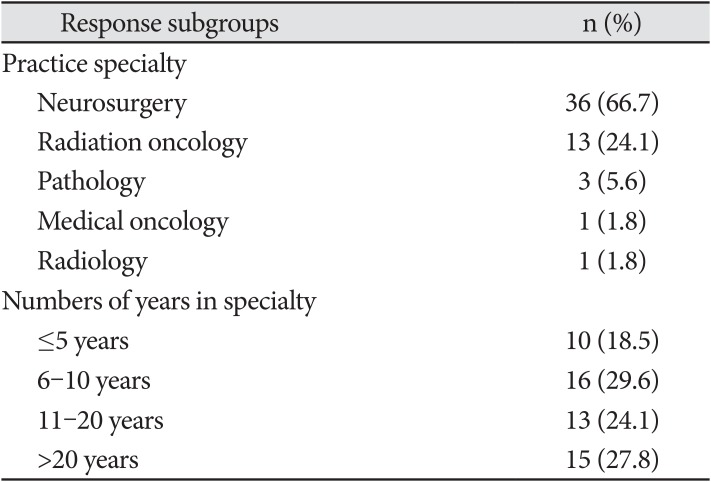




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download