INTRODUCTION
Tumor-to-tumor metastasis (TTM) is defined as the hematogenous metastasis within a primary host tumor from a donor neoplasm. Although the precise mechanism of TTM is still unclear, it has been reported in the literature for over a century [
1]. However, metastasis of a tumor to an intracranial meningioma has been rarely reported [
2]. According to the literature, lung cancer and breast cancer are the most common donor tumors in TTM [
234]. Among host tumors, renal cell carcinoma, thyroid carcinoma, and adrenocortical adenoma are common. The most common intracranial host tumors include meningioma, pituitary adenoma, schwannoma, glioma, and hemangioblastoma [
4].
Here, we present our experience with a case of TTM of breast cancer to meningioma as well as a review of literature to provide a better understanding of this rare phenomenon.
CASE REPORT
A 73-year-old female patient who underwent neoadjuvant chemotherapy for six months for Nottingham histologic grade 2 invasive ductal carcinoma of the breast was referred to the neurosurgical department at our institution. She was incidentally diagnosed with a parasagittal meningioma in the right frontal area on MRI, which was performed for a systemic workup (
Fig. 1). Brain MRI revealed a well-circumscribed strong enhancing mass of 3.4 cm with minimal peritumoral edema. She was followed up in the outpatient clinic, and the size of the tumor and peritumoral edema was found to have increased on MRI 16 months later (
Fig. 2). Subsequently, surgical resection was planned, and frontal craniotomy with complete removal of the parasagittal meningioma was performed (
Fig. 3). The lesion was resected
en bloc along with the removal of the adjacent superior sagittal sinus and falx.
Fig. 1
Brain MRI in a 73-year-old female patient shows strongly enhancing 3.4-cm parasagittal meningioma (A) with mild peritumoral edema on axial T2-weighted MRI (B).
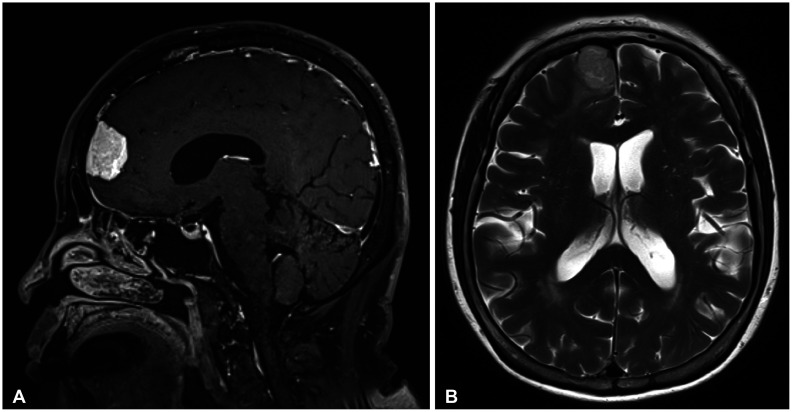

Fig. 2
Follow-up MRI 16 months later shows increased tumor size (A) and peritumoral edema (B).
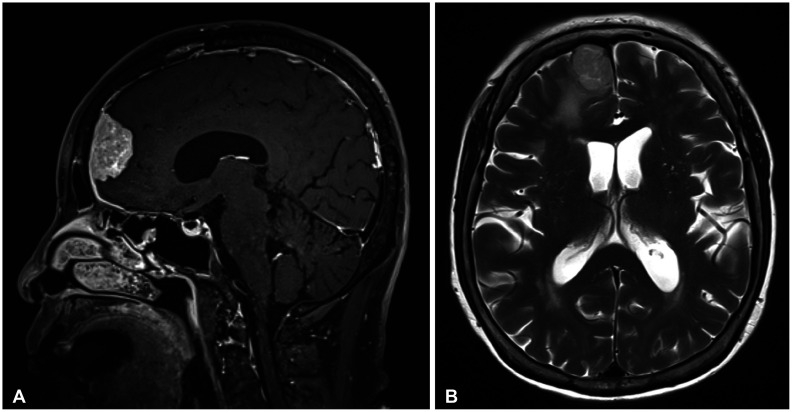

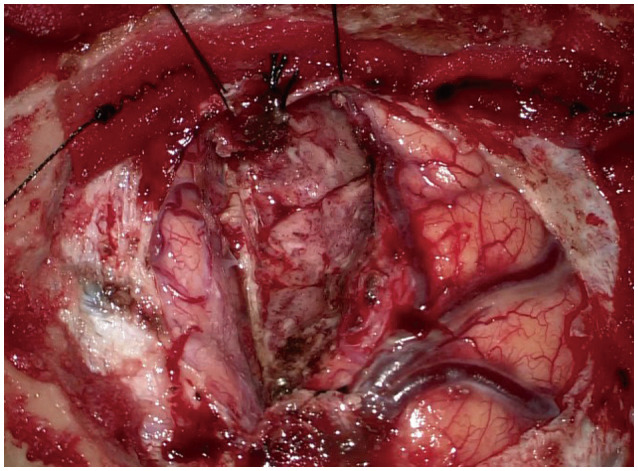
Fig. 3
Intraoperative surgical view showing complete removal of the tumor with the adjacent superior sagittal sinus and falx.

Histopathological studies confirmed a WHO grade 1 secretory meningioma, which revealed a meningothelial structure with positive staining for somatostatin receptor 2a, carcinoembryonic antigen, and cytokeratin in the pseudo-psammomatous bodies (
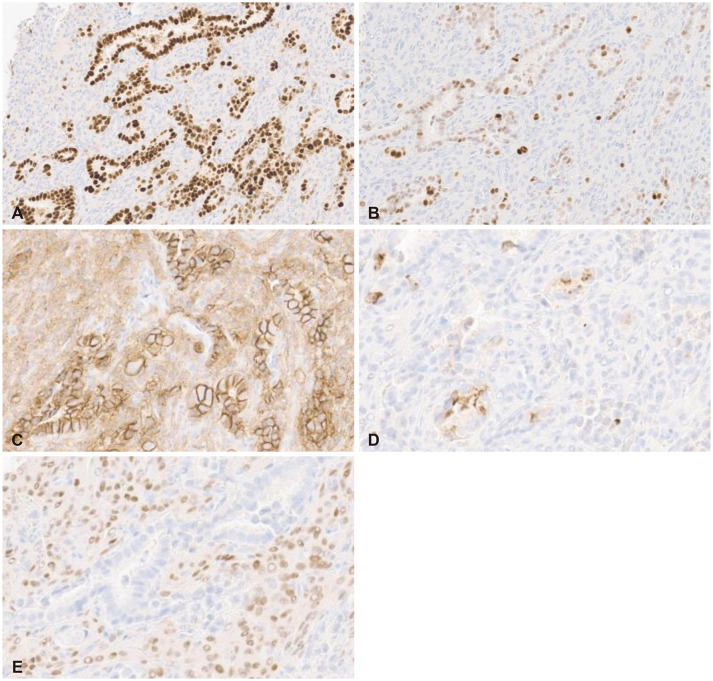
Fig. 4). Furthermore, a small nest of focally metastatic carcinoma deposits was noted within the meningioma. The carcinoma components revealed nuclear immunoreactivity to GATA binding protein 3 and estrogen receptor (ER), membranous positivity to cytokeratin and E-cadherin, focal cytoplasmic positivity to gross cystic disease fluid protein 15, and negative immunoreactivity to progesterone receptor (PR), which was consistent with a diagnosis of epithelial ductal carcinoma (
Fig. 5). Therefore, a diagnosis of TTM of breast cancer to the meningioma was established.
Fig. 4
Histopathologic findings of the meningioma components show sheets of meningothelial cells (A) and pseudo-psammomatous bodies inside a gland-like space (B) with immunopositivity for somatostatin receptor 2a (C) and carcinoembryonic antigen, cytokeratin (inlet) in the pseudo-psammomatous bodies (D). A and B: H&E stain, original magnification, ×200; C and D: Immunohistochemistry, original magnification, ×200.
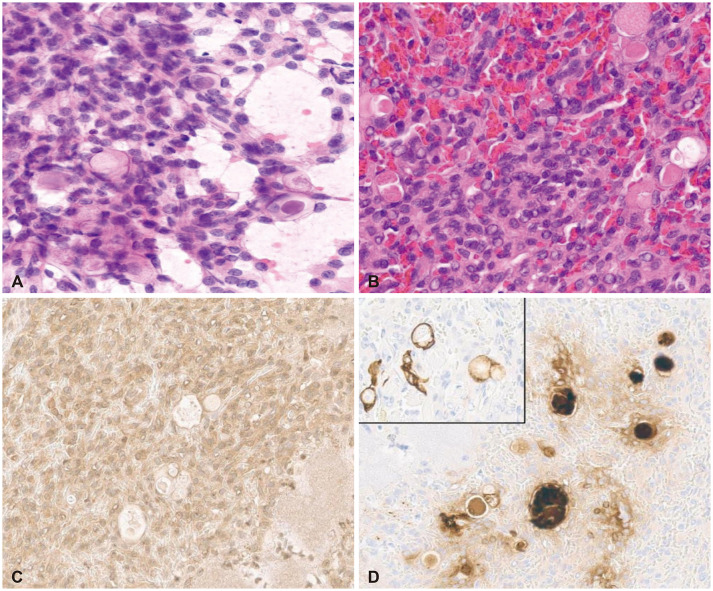

Fig. 5
Histopathologic findings of the carcinoma components show nuclear immunoreactivity of GATA binding protein 3 (A) and estrogen receptor (B), membranous positivity of E-cadherin (C), focal cytoplasmic positivity of gross cystic disease fluid protein 15 (D), and negative immunoreactivity of progesterone receptor, which are consistent with breast ductal carcinoma (E). A-E: Immunohistochemistry, original magnification, ×200.

DISCUSSION
TTM refers to tumor cells that metastasize via the blood to a primary host tumor (recipient tumor) from a donor tumor [
4]. To establish a diagnosis of TTM, the donor tumor must be partially surrounded by the host tumor and metastasis of the primary tumor must be present. It is sometimes confused with a collision tumor, which possesses similar characteristics as TTM. It is crucial to differentiate TTM from a collision tumor, which refers to the presence of two histologically distinct tumors within an anatomic location with infiltration of the tumors into one another [
567]. Syed et al. [
7] reported that understanding the distinction between a collision tumor and TTM is necessary to plan the surgical approach and management.
There are several criteria to diagnose TTM and differentiate it from a collision tumor (
Tables 1 and
2) [
89]. According to Campbell et al. [
8], TTM can be diagnosed when the following conditions are satisfied: 1) coexistence of two or more primary tumors; 2) the host tumor must be a true neoplasm; 3) histologically different tumor tissue must enclose the metastatic focus, not be of contiguous growth, with the absence of an intermediate transitional zone; and 4) extravascular metastasis must be present with the exclusion of metastasis to the lymphatic system (such as lymphoma) where the second primary tumor is present with an active hematologic malignancy (
Table 1). Additionally, the criteria proposed by Richter et al. [
9] require at least a partial enclosure of the metastatic focus from a histologically different host tumor with a proven primary tumor with histological compatibility (
Table 2).
Supplementary Table 1 (in the online-only Data Supplement) demonstrates the previously reported TTM cases classified according to the host tumor.
Table 1
Proposed criteria for tumor-to-tumor metastasis

|
Proposed diagnostic criteria by Campbell et al. [8] |
|
1. At least two primary tumors must exist |
|
2. The host tumor must be a true neoplasm |
|
3. The metastatic focus must show established growth inside of the host tumor, must not be the result of contiguous growth |
|
4. The host tumor cannot be a lymph node involved by leukemia or lymphoma |

Table 2
Proposed criteria to differentiate tumor-to-tumor metastasis (TTM) with collision tumor

|
Proposed differentiation criteria between TTM and collision tumor by Richter et al. [9] |
|
1. The metastatic focus must be at least partially enclosed by a rim of histologically distinct host tumor tissue |
|
2. The presence of the metastasizing primary carcinoma must be proven and compatible with the metastasis |

According to the literature, lung cancer and breast cancer are the most common donor tumors [
34]. Additionally, various intracranial tumors, such as meningioma, hemangioblastoma, and pituitary adenoma can be the host tumors with meningioma being the most common intracranial host tumor [
4]. The overall incidence of TTM is still unknown. Hu et al. [
10] reported their 2,922 and 540 consecutive patients diagnosed with meningioma and intracranial metastasis, respectively, with only 1 TTM patient identified, accounting for 0.03% of meningioma and 0.19% of intracranial metastasis.
Several pathophysiological mechanisms have been proposed to clarify why a meningioma can be the most frequent host tumor [
27111213]. Since most meningiomas are benign and slow-growing tumors, the survival rate is higher along with the risk of intratumoral metastasis [
1114]. Additionally, a meningioma is a highly vascularized tumor, which is another critical factor that predisposes it to metastasis [
12]. In general, meningiomas have high collagen and lipid content that constitute an ideal environment for implantation, thus making them vulnerable to become a recipient tumor according to Paget’s “soil and seed” hypothesis [
2]. The hypothesis states that the high collagen and lipid content in meningiomas are likened to “fertile soil” in which seeds of malignant cells thrive [
12]. Furthermore, according to Fukushima et al. [
15], meningiomas have a low metabolic rate, which creates a favorable environment for tumors to grow with less competition for metastatic tumor growth. Similarly, intracranial tumors lack an immune response, which might help in the proliferation of metastasis in intracranial tumors [
1416]. Additionally, the presence of intercellular communication factors, such as E-cadherin, which plays a role in cell adhesion, may be one of the pathophysiological mechanisms involved in TTM, particularly in TTM of ductal breast carcinoma to meningioma [
13]. Lastly, ER and PRs expressed by breast carcinoma and meningioma might be involved in cell-to-cell interactions as well [
17].
Depending on the location, TTM can result in various neurological symptoms, such as loss of consciousness and hemiplegia [
18]. Most intra-meningioma metastases are diagnosed histopathologically, and they cannot be accurately diagnosed using CT or MRI. Furthermore, it is difficult to differentiate TTM from a collision tumor using MRI [
5]. However, MRI may be used to identify rapid growth, peritumoral edema, irregular margins, mushroom pattern, and heterogeneous enhancement, which have been reported previously [
713]. Some studies have reported that perfusion MRI and magnetic resonance spectroscopy might be helpful in diagnosing TTM and differentiating it from a meningioma [
1920]. Dietterle et al. [
21] reported that a malignant tumor or TTM should be suspected in a tumor in a patient who has been symptom-free for many years if it suddenly increases in size along with neurological deterioration.
Until now, there is no sufficient evidence how an intra-meningioma metastasis affects the clinical course, treatment and their prognosis. The treatment of TTM is surgical removal to minimize the potential spread of malignant cells, confirm the histologic diagnosis, and decrease the effects of an intracranial mass. Postoperative adjuvant therapy may be required [
4]. Intracranial metastasis can be parenchymal, leptomeningeal, or both. In cases of leptomeningeal carcinomatosis, intrathecal chemotherapy may be also considered. In general, the prognosis of leptomeningeal carcinomatosis is considered poor. The prognosis can be more favorable in case of parenchymal metastasis. However, it is unfeasible to designate intramenigioma metastasis into certain form of metastasis, and the management should be opted depending on each case [
4].
In conclusion, we presented a rare case of TTM of breast cancer into a meningioma, which was treated using surgical resection. It is important to consider the possibility of TTM in a patient with systematic malignancy in the setting of a meningioma. The clinical relevance of TTM remains unclear, and future studies are needed to clarify the optimal management and prognosis of this rare phenomenon.








 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download