Abstract
Background
Methods
Results
Notes
Author Contributions:
Conceptualization: Eui Hyun Kim.
Data curation: Ginam Kim.
Formal analysis: Ginam Kim.
Funding acquisition: Eui Hyun Kim.
Investigation: Ginam Kim, Ju Hyung Moon, Sun Ho Kim, Eui Hyun Kiim.
Project administration: Eui Hyun Kim.
Resources: Eui Hyun Kim.
Software: Ginam Kim.
Supervision: Eui Hyun Kim.
Validation: Eui Hyun Kim.
Visualization: Eui Hyun Kim.
Writing—original draft: Ginam Kim.
Writing—review & editing: Eui Hyun Kim.
Funding Statement: This study was funded by the Basic Science Research Program through the NRF of Korea (2021R1F1A1051996) from the Korean Ministry of Science, ICT and Future Planning (Eui Hyun Kim) and the Team Science Award Grant of Yonsei University College of Medicine (6-2021-0009) (Eui Hyun Kim).
Availability of Data and Material
References
Fig. 1
The characterization of magnetic resonance imaging (MRI) signals of cyst contents in both T1W and T2W images. Among possible nine combinations, we identified four major groups based on MRI signal characteristics; Type S-high, Type S-iso, Type S-low and Type M.
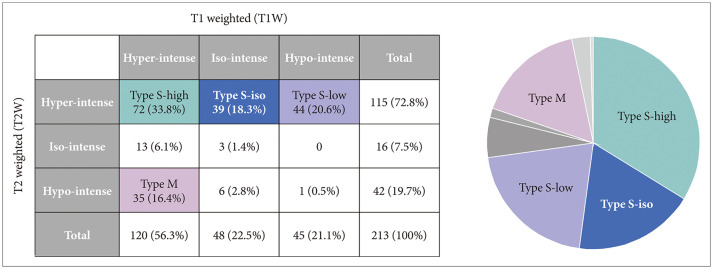
Fig. 2
Magnetic resonance images (MRI) of four representative cases of Rathke’s cleft cyst (RCC). A and B: Type S-high. A 44-year-old female patient with RCC underwent transsphenoidal surgery for visual disturbance. The MRI shows a hyperintense signals both in T1-weighted (T1W) and T2-weighted (T2W) sagittal images. C and D: Type S-iso. A 45-year-old male patient was just followed-up without surgical intervention for 5 years. MRI revealed Rathke’s cleft cyst with hypointensity in T1W and hyperintensity in T2W. E and F: Type S-hypo. A 44-year-old female patient with RCC was followed-up for 7 years without surgical treatment as the size of the cyst did not change. The MRI revealed hypointense signal in T1W and hyperintense signal in T2W image. G and H: Type M. A 32-year-old female patient underwent surgery for relieving intractable headache. Preoperative MRI revealed hyperintensity in T1W and hypointensity in T2W images.
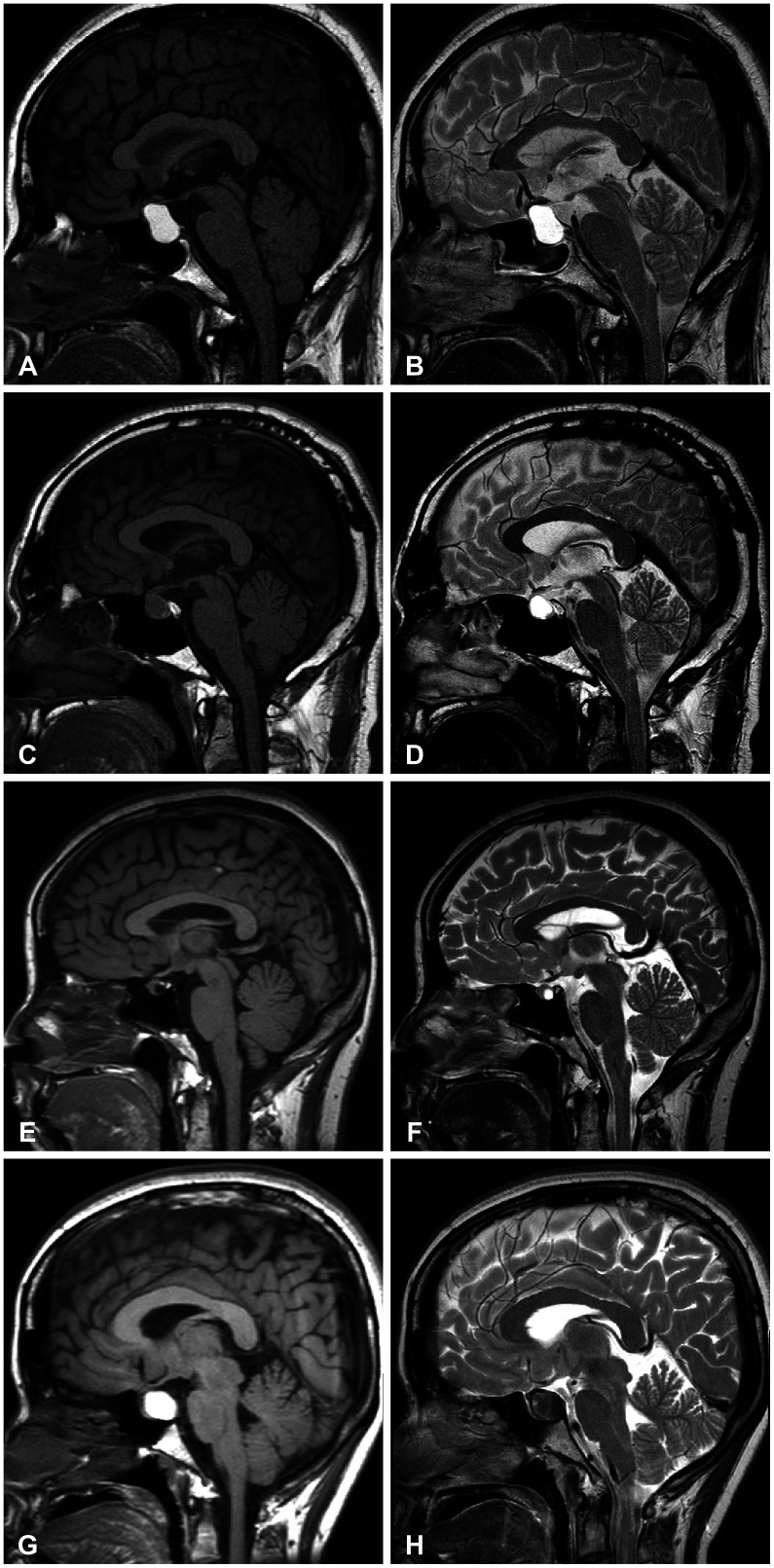
Fig. 3
Comparison between observation (A) and surgical (B) groups. Comparative analysis revealed that only two major groups in which the number of patients in the surgical and observation groups was statistically different: more Type S-low in the surgical group and more Type M in the observation group. *p-value<0.001; †p-value=0.007.
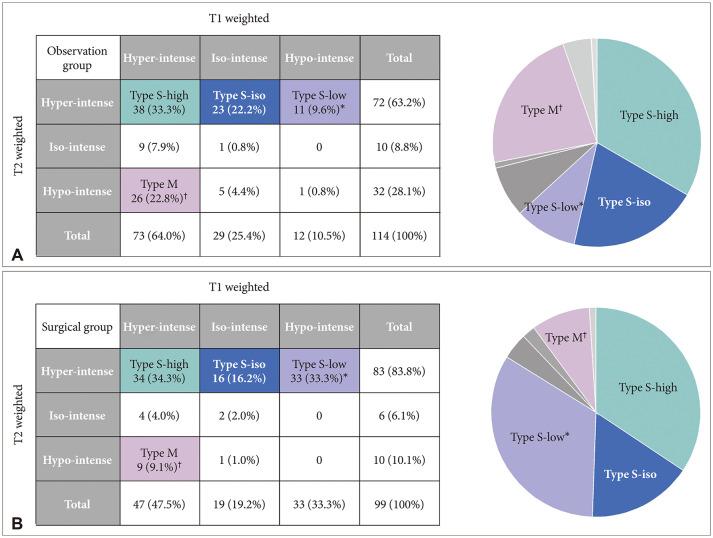
Fig. 4
Visual and endocrine outcomes in the surgical group. A: In the surgical group, the main indications of surgery were visual deterioration in 41 patients, endocrine hormone deficiency in 29 patients, and other reasons such as uncontrolled headache in 22 patients. The Type S-low group included more patients who underwent surgery for visual compromise. B and C: Visual improvement was achieved in 26 patients (83.9%), whereas visual function did not change in 2 patients and worsened in 3 patients while endocrine function was improved in 16 patients (72.8%), worsened in 5 patients (22.7%), and did not change in 1 patient after surgery. However, the intergroup differences in visual and endocrine outcomes were not evident. *p-value=0.034.
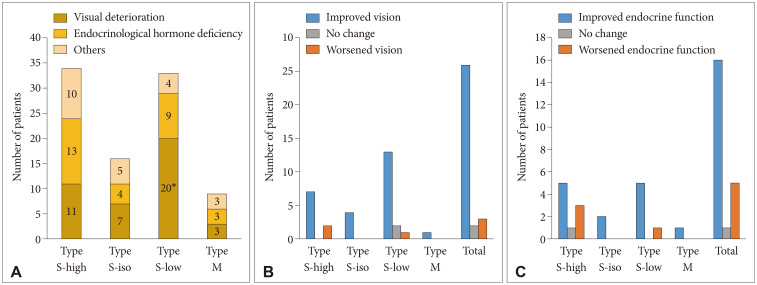
Table 1
Clinical characteristics of patients with Rathke’s cleft cyst
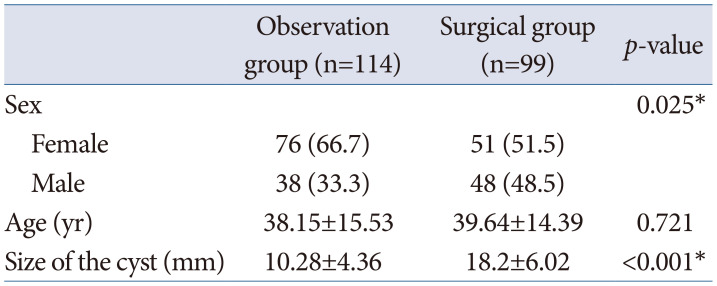
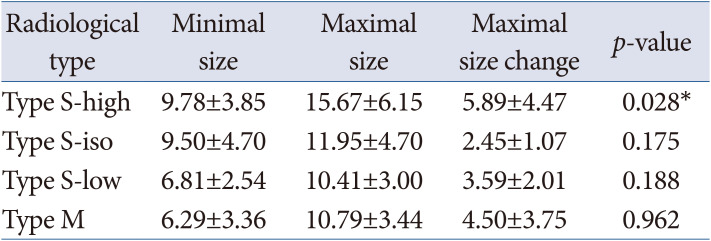
Table 2
Size of the Rathke’s cleft cyst during follow-up in the observation group




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download