Abstract
A 41-year-old man suffered from progressive radiculomyelopathy caused by spinal epidural mass primarily encasing the spinal cord at the cervicothoracic vertebrae that extended into the thoracic cavity through the neural foramen. An urgent decompressive laminectomy and epidural tumor resection were performed to prevent neurological deterioration and effective spinal cord decompression. The histopathologic diagnosis was diffuse large B-cell lymphoma. As first-line treatment for stage II extranodal lymphoma, he received 6 cycles of R-CHOP (rituximab/cyclophosphamide, hydroxydaunorubicin, Oncovin, and prednisone) chemotherapy. Consequently, follow-up positron-emission tomography CT and MR images demonstrated a complete metabolic response (Deauville score 1). This rare occurrence of primarily extranodal spinal epidural lymphoma with limited disease will be presented in a literature review.
Lymphomas account for 5% of all adult systemic cancer [1]; spine lesions seldom occur in 5.8%–6.5% of lymphoma [23]. Spinal lymphoma is relatively uncommon and accounts for 1%–2% of the overall extranodal occurrence of systemic lymphoma [4]. Spinal lymphoma usually developed advanced and systemic disseminated stage as metastatic lesions. Only 0.1%–6.5% of all lymphomas are present in epidural space [5].
Since extranodal epidural lymphoma occurs rarely in both Hodgkin’s and non-Hodgkin’s types, the diagnosis of the disease is challenging. Extranodal and primary spinal epidural lymphoma (PSEL) is an extremely rare entity of lymphomas located in the epidural without any other recognizable sites of lymphomas at diagnosis [5]. However, with recent advances in more sensitive imaging modalities, the incidence of diagnosis has increased. Non-Hodgkin’s lymphomas are often found to be a common form of extranodal origin (approximately 24%–48%). The most common histology is diffuse large B-cell lymphoma (DLBCL) and prevalence according to the location is in the order of thoracic, lumbar, and cervical spine [6].
Herein, we are to present a case of a rare occurrence of primarily multilevel extranodal spinal epidural lymphoma with progressive spinal cord compression and adjacent thoracic invasion that was eventually diagnosed as DLBCL.
A 41-year-old man suffered from progressive intolerable pain on the posterior neck, upper thoracic back radiating to both shoulder, left-hand weakness, and numbness of 4th-5th fingers for 2 months. The symptoms began acutely with paresthesia and a tingling sensation during playing golf. His symptoms had not improved with conservative treatments including physiotherapy and acupuncture as well as percutaneous procedures for local pain control. Once his pain got worsened and aggravated gradually, he visited our emergency room with intolerably severe neck and upper thoracic pain and progressive left arm weakness including numbness of both hands. On admission, he had severe interscapular and neck pain (visual analogue scale=8–9). Neurological examinations revealed left arm weakness of elbow, wrist, and grasping power in grade 3.
MRI images demonstrated a contrast-enhancing lesion in the epidural space which compressed and encased the spinal cord in the spinal canal from C4 to T5. The epidural mass was extending into bilateral intervertebral foraminal spaces to spread to the left pleural space diffusely with pleural thickening. There was also an enhanced lesion in the epidural space with bilateral foraminal involvement, the right side of C6-T3 and the left side of C5-T6 (Fig. 1). Positron-emission tomography (PET) CT showed that a mass of more than 3 cm size with strong fluorodeoxyglucose (FDG) uptake in the anterior mediastinum, multiple enlarged lymph nodes in the left supraclavicular portion and left mediastinum (suggesting lymph node metastasis), multiple plaque-like lesions in the left pleura with strong FDG uptake suggesting pleural metastasis (Fig. 2). For pathological diagnosis and spinal cord decompression, he underwent elective surgical resection of the tumor encasing the upper thoracic spinal cord via the decompressive laminectomy T1, 2, 3, 4 (Fig. 3). The mass compressed spinal cord was a consecutive avascular band-like mass with the finding of reactive inflammation. Post-operatively, both hand weakness with severe upper thoracic axial and radiating pain was remarkably resolved. Two weeks after the surgery, it had improved to motor power grade 5.
Histological findings demonstrated diffuse proliferation of atypical small round cells, which were large cells with scanty cytoplasm and basophilic or vesicular nuclei on H&E staining (×200) (Fig. 3). Immunohistochemistry findings showed a 40% of Ki-67 proliferative index and strong positive CD20 as a B-cell marker (Fig. 4). All other markers, however, including CD3, CD10, CD30, Bc12, Bc16, C-myc, and MUM1 (multiple myeloma oncogene-1) were negative. The histologic diagnosis was DLBCL. Then the patient was transferred to the hematooncologic department for staging work-up and subsequent chemotherapy. A systematic investigation determined Stage II E extranodal spinal epidural malignant lymphoma that indicated non-bulky type (<7.5 cm), two or more nodal involvements on the same left side of the diaphragm consistent with mediastinal malignancy with pleural metastasis. There were no definite abnormal glucose metabolic lesions in the brain and currently, no remarkable finding is observed in the abdominopelvic cavity on CT image. Also, there was no abnormality in the hepatobiliary, gastrointestinal tract, including the pancreas and spleen, and genitourinary tract as well as contralateral lung and mediastinum.
According to National Comprehensive Cancer Network (NCCN) guidelines, chemo port was inserted on the right subclavian vein (Fig. 4) for chemotherapy. The patient responded effectively to the primary 6 cycles of adjuvant R-CHOP (rituximab/cyclophosphamide, hydroxydaunorubicin, Oncovin, and prednisone) chemotherapy. After 3 cycles of R-CHOP chemotherapy, PET CT was performed for interim staging that showed the near complete remission of lymphoma lesions in the left pleura and mediastinum. Consequently, he showed a complete metabolic response (Deauville score 1) after the primary 6 cycles of R-CHOP chemotherapy during the follow-up period. The chemoport was removed after the confirmation of complete remission in imaging studies (Fig. 5). The patient is under outpatient follow-up as the symptoms have disappeared and muscle strength has completely recovered.
Extranodal spinal epidural lymphoma is a rare condition characterized by DLBCL and spinal cord compression, Spinal cord compression is one of the most common and serious neurological manifestations seen in spinal lymphoma.
By definition, PSEL refers to lymphoma without other disease sites at the time of diagnosis and the frequency of occurrence accounts for about 0.1%–0.6% of all lymphoma [5]. At the time of diagnosis, spinal MRI findings of this case showed characteristic features of PSEL as initial presentation, however, during staging work up, we found that local spread of disease into the unilateral thoracic cavity and lymph node. Therefore, we proposed that this occurred local invasion from PSEL. In general, metastatic spinal epidural lymphoma indicates a diffusely enhancing epidural invasion, which extends to both ventrally and dorsally on the length of the spinal canal with disseminated bony invasion. In addition, metastatic lesions typically occurred advanced and late stage of systemic lymphoma, unlike this case. Therefore, we assumed that this case presented extensive multilevel involvement of PSEL with local invasion through the intervertebral foramina at the time of diagnosis. Therefore, we reported this case as a rare occurrence of primarily extranodal spinal epidural lymphoma. The PSEL originates from lymphatic tissue along the venous plexus in the epidural space of the paravertebral lymphoid rests and originates from paraspinal, vertebral, and retroperitoneal tissue after entering through the intervertebral foramina.
Systemic staging work-up indicates determining the therapeutic regimen and strategy. In terms of NCCN guidelines for lymphoma, 6 cycles of neoadjuvant R-CHOP Chemotherapeutic regimes and precise delivery techniques of radiotherapy act as the key disease control methods. Surgery is the cornerstone of tissue confirmation and urgent surgical intervention for spinal cord compression followed by adjuvant chemotherapy or radiotherapy that increases the disease-free survival period. Approximately, 50% of patients survive more than 3 years who undergo potentially curable diseases with good clinical outcomes when treated with surgery and multimodality treatment [7]. The therapeutic outcome of spinal cord compression caused by extranodal spinal epidural lymphoma is far better in regards to both functional recovery and survival than that of metastatic spinal cord compression from other systemic cancer. According to a 2006 multicenter rare cancer network study, the 5-year survival rate increased significantly to 75% and 61% of disease-free survival period for combined therapy rather than radiotherapy alone. In a report that included the analysis of 94 papers in China for 30 years in 2016, the prognosis of combined treatment of surgery, radiotherapy and chemotherapy, and surgery plus chemotherapy was analyzed to have a 3-year survival rate of about 80% and a 3-year disease-free survival rate of approximately 50% [89].
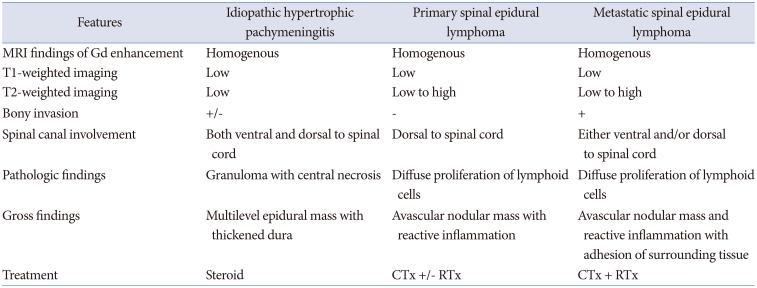
As in this case, the patient underwent surgery for the adequate and effective resection of the epidural spinal cord compression. According to the pathologic confirmation, 6 cycles of adjuvant R-CHOP chemotherapy were completed and which showed a complete response (Deauville score 1). Eventually, there was no evidence of relapse and excellent functional outcomes during the follow-up period. It is important to exclude the differential diagnosis of this rare disease entity as a mimetic pathology characterized by multilevel consecutive pure epidural lesions surrounding the spinal cord encased. Preoperative radiographic diagnosis is very challenging to differentiate the similarity of these rare disease entities invading the multilevel continuous epidural involvement with MRI findings only. However, histopathologically, the extranodal and PSEL can be characterized by diffuse proliferation of lymphoid cells, and avascular nodular mass with reactive inflammation. Unlike PSEL, inflammatory pseudotumors, such as idiopathic hypertrophic pachymeningitis often characterized by granuloma with central necrosis, and similarly multilevel consecutive epidural inflammatory mass with thickened dura (Table 1) [10].
There may be some debates as to whether this spinal epidural lymphoma is primary or secondary. However, metastatic spinal epidural invasion of systemic lymphoma usually occurs in the late stage of the advanced disease. In addition, the metastatic growth demonstrates a diffusely enhancing epidural invasion extending both ventrally and dorsally on the length of the spinal canal with disseminated bony invasion unlikely in this case. Often, intradural invasion of cerebrospinal fluid seeding is a common characteristic finding of advanced systemic lymphoma [1112]. In MRI findings, extranodal and PSEL show a low signal at T1-weighted imaging (WI) and low to high signal intensity at T2WI and are characterized by no bony invasion in the spinal canal. In contrast, metastatic spinal lymphoma shows a more disseminated invasion of both epidural and intradural spinal cord and bony infiltration of the spinal column (Table 1). We hypothesize that the PSEL was locally spread to the adjacent region by direct invocation during consecutive periodic acupuncture and percutaneous pain procedures in the treatment of his axial spinal and radicular pain. It is important to accurately diagnose and treat the unexplained cause of pain with MRI before invasive treatment. On the other hand, MR finding of idiopathic pachymeningitis typically demonstrates that the tumor shows a low signal in T1 and T2WI, with or without bony involvement and both ventral and dorsal infiltration of the spinal cord. It is mainly treated in response to medication such as steroids. From a point of view of surgical strategy, biopsy allows for histopathological diagnosis in patients with no other known diagnosis related to the epidural lesion and planning of the chemotherapy and radiotherapy. Surgery is the mainstay of treatment for cord compression with chemotherapy and/or radiotherapy and it is associated with good functional outcomes. Surgery alone is not to be effective at patients’ median overall survival.
PSEL is a potentially curable disease with good clinical outcomes and prognosis when diagnosed early and treated with surgery and multimodality treatment. There are two pathophysiological hypotheses of this disease that originated from the lymphoid tissue of venous flexus inside the paravertebral lymphoid rest and originated from paraspinal, vertebral, and retroperitoneal tissue spread along the intervertebral foramen [781314]. In this case, it is believed to have been spread along the intervertebral foramen of the cervical and thoracic segments originating from paraspinal lymphoid tissue and pleural tissue. PSEL should be suspected in patients with spinal cord compression and with imaging showing isointense, homogeneous extradural continuous level compressive soft-tissue lesions bony involvement, and previous history of cancer. Chemotherapeutic regimes and precise delivery techniques of radiotherapy act as the key disease control methods. Surgery is the cornerstone of treatment for cord compression with chemotherapy and radiotherapy, and is associated with good functional outcomes. Adjuvant therapy increases the disease-free survival period. Multi-modality treatment including chemotherapy and/or radiotherapy and surgery is optional in the case of spinal core compression and the prognosis of PSEL is far better in terms of both functional recovery and survival than that of cord compression in metastatic cancer [15].
In conclusion, although these diseases are rare, it is recommended to be aware of the possibility of occurrence of this disease, along with careful medical history and appropriate early imaging diagnosis, and of the characteristic imaging findings and disease progression with therapeutic principle. This is because early diagnosis of this disease has a great impact on the quality of life and sequelae of patients.
Notes
Ethics Statement: Ilsan Paik Hospital, College of Medicine, Inje University IRB (IRB number: 2022-06-013).
Author Contributions:
Conceptualization: Moon-Jun Sohn, Min-Cheol Seok.
Data curation: Min-Cheol Seok, Ahmad Khalid Madadi, Mohammad Mohsen Mosleh.
Investigation: Min-Cheol Seok, Sun Hee Chang, Ahmad Khalid Madadi, Mohammad Mohsen Mosleh.
Supervision: Moon-Jun Sohn.
Visualization: Min-Cheol Seok, Sun Hee Chang, Ahmad Khalid Madadi.
Writing—original draft: Min-Cheol Seok, Ahmad Khalid Madadi.
Writing—review & editing: Moon-Jun Sohn.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Hashi S, Goodwin CR, Ahmed AK, Sciubba DM. Management of extranodal lymphoma of the spine: a study of 30 patients. CNS Oncol. 2018; 7:CNS11. PMID: 29706086.

2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022; 72:7–33. PMID: 35020204.

3. Uehara M, Takahashi J, Hirabayashi H, Kitahara J, Kamijyo T, Ebara S, et al. Hodgkin’s disease of the thoracic vertebrae. Spine J. 2013; 13:e59–e63.

4. Koeller KK, Shih RY. Extranodal lymphoma of the central nervous system and spine. Radiol Clin North Am. 2016; 54:649–671. PMID: 27265601.

5. Cugati G, Singh M, Pande A, Ramamurthi R, Balasubramanyam M, Sethi SK, et al. Primary spinal epidural lymphomas. J Craniovertebr Junction Spine. 2011; 2:3–11. PMID: 22013369.

6. Özdemir NG, Baran Ö, Şahin ÖF, Akbaş F, Köker HT, Huq GE. Primary spinal epidural lymphoma: a case report. Istanb Med J. 2020; 21:32–35.

7. Mally R, Sharma M, Khan S, Velho V. Primary lumbo-sacral spinal epidural non-Hodgkin’s lymphoma: a case report and review of literature. Asian Spine J. 2011; 5:192–195. PMID: 21892393.

8. Monnard V, Sun A, Epelbaum R, Poortmans P, Miller RC, Verschueren T, et al. Primary spinal epidural lymphoma: patients’ profile, outcome, and prognostic factors: a multicenter rare cancer network study. Int J Radiat Oncol Biol Phys. 2006; 65:817–823. PMID: 16542791.

9. Xiong L, Liao LM, Ding JW, Zhang ZL, Liu AW, Huang L. Clinicopathologic characteristics and prognostic factors for primary spinal epidural lymphoma: report on 36 Chinese patients and review of the literature. BMC Cancer. 2017; 17:131. PMID: 28196505.

10. Park SH, Whang CJ, Sohn M, Oh YC, Lee CH, Whang YJ. Idiopathic hypertrophic spinal pachymeningitis: a case report. J Korean Med Sci. 2001; 16:683–688. PMID: 11641545.

11. Luo CC. Spinal cord compression secondary to metastatic non-Hodgkins lymphoma: a case report. Arch Phys Med Rehabil. 2005; 86:332–334. PMID: 15706563.

12. Yáñez ML, Miller JJ, Batchelor TT. Diagnosis and treatment of epidural metastases. Cancer. 2017; 123:1106–1114. PMID: 28026861.

13. Jagtap S, Patil A, Kesavdas C, Radhakrishnan N, Soni H, Satish K. Primary spinal epidural diffuse large B-cell lymphoma. Neurol India. 2013; 61:532–534. PMID: 24262463.

14. Kim SK, Lee SH, Kim ES, Eoh W. Diffuse large B-cell lymphoma mimicking schwannoma of lumbar spine. Korean J Spine. 2016; 13:71–73. PMID: 27437017.

15. Boukobza M, Mazel C, Touboul E. Primary vertebral and spinal epidural non-Hodgkin’s lymphoma with spinal cord compression. Neuroradiology. 1996; 38:333–337. PMID: 8738091.

Fig. 1
Preoperative MRI findings. A-C: The fat suppression with gadolinium (Gd) enhancement showed that consecutive epidural mass extended into both intervertebral foramina from C3 to T7 vertebrae lesions (white arrows) on sagittal and coronal sections. D and E: The spinal cord was encased by diffuse homogenously enhancing mass lesions (white arrows) on axial T1-weighted imaging with Gd enhancement at T1 and T4 vertebra.
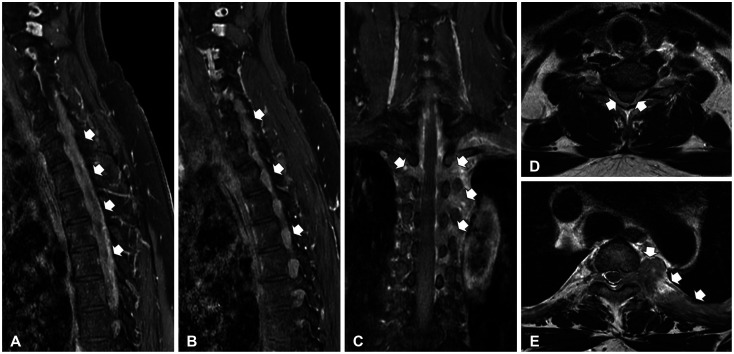
Fig. 2
PET CT scan on staging workup. A and B: PET-CT indicates that extranodal spinal epidural lymphoma extended into the unilateral mediastinal cavity through the left pleura spread (sagittal and coronal image, white arrows). C and D: The multiple lymph node invasion in the left supraclavicular (C, white arrows) and mediastinal lymph nodes along with ribs suggests metastasis (D, white arrows). PET, positron-emission tomography.
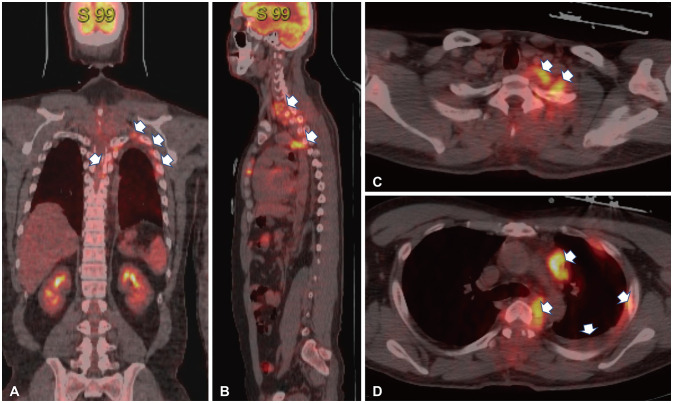
Fig. 3
Intraoperative photos of initial and surgical resection and histologic features. A and B: Upon exposing the spinal cord, avascular amorphous encasing mass was continuously compressed the spinal cord. C: Following adequate surgical decompression, the spinal cord was satisfactorily expanded from tumor decompression. D: On histologic findings, H&E staining (×200) demonstrates atypical round-shaped large cells in scanty cytoplasm with basophilic or vesicular nuclei that are compatible with diffuse large B-cell lymphoma.
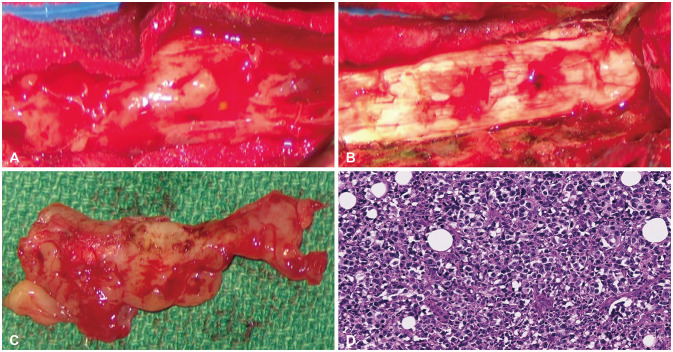
Fig. 4
Immunohistochemistry findings and chemoport insertion. A and B: Immunohistochemistry findings reveal a high Ki-67 proliferative index of 40% (A) and a strong positive CD20 as a B-cell marker (B). C: Chemoport was inserted into the right subclavian vein for R-CHOP (rituximab/cyclophosphamide, hydroxydaunorubicin, Oncovin, and prednisone) chemotherapy.
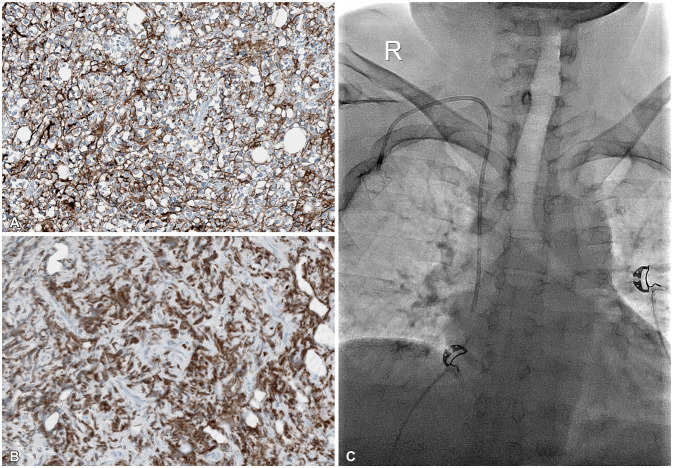
Fig. 5
Radiographic imaging follow-up after 6 cycles of R-CHOP chemotherapy. A and B: Follow-up MR image of fat suppression Gd enhancement demonstrates complete radiographic and metabolic response (Deauville score 1) at 6 months. C and D: PET scan images also define initial extranodal spinal epidural lymphoma with left supraclavicular and mediastinal lymph node invasion that has completely disappeared. R-CHOP, rituximab/cyclophosphamide, hydroxydaunorubicin, Oncovin, and prednisone; Gd, gadolinium; PET, positron-emission tomography.
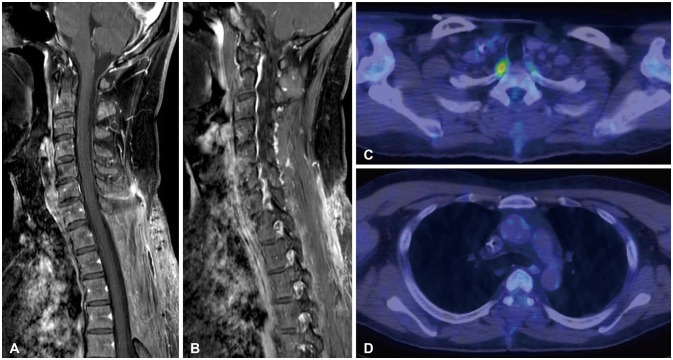
Table 1
Differential diagnosis of primary spinal epidural lymphoma




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download