Abstract
During the last three decades, the management of medulloblastoma (MBL) has made enormous progress with a multidisciplinary approach, incorporating surgery, radiotherapy (RT), and chemotherapy. Despite this improvement, 20%–30% of patients with MBL remain at risk of disease recurrence, with its relapse being possibly fatal. To date, the salvage treatment for relapse remains challenging, and various approaches have been suggested for the retreatment. In this review, I have described the characteristics of patients with relapsed MBL, patterns of relapse and the most commonly prescribed treatment. Further, I have reviewed the studies on re-irradiation and its associated issues to conclusively suggest the RT recommendations for patients with relapsed MBL.
Medulloblastoma (MBL) is the most common malignant brain tumor in children and has a propensity of leptomeningeal seeding. Therefore, after surgical resection, whole craniospinal axis should be the target of radiotherapy (RT) [12]. The management of MBL has made enormous progress over the last three decades with a multidisciplinary approach, and the 5-year survival rates are approximately 85% and 70% in standard- and high-risk patients, respectively [3456]. However, approximately 20%–30% of patients with MBL remain at risk of disease recurrence, and they have not responded to the salvage therapy, with a dismal prognosis, and are universally fatal [37891011].
To date, the salvage treatment for relapse remains challenging and several approaches including second surgical resection, chemotherapy such as multi-agent chemotherapy or high-dose chemotherapy (HDCT) with autologous stem cell transplantation, and RT including re-irradiation have been suggested. And lately, some clinical studies using targeted therapy have been reported.
In regard to RT, some studies have demonstrated the potential effectiveness as a salvage treatment for relapsed MBL in infant patients, who were previously treated with a radiation-sparing regimen [121314]. On the other hand, considerable debate exists about the improvement of treatment outcomes with re-irradiation by weighting it against the potential increase in toxicity and uncertainty of survival improvement. Furthermore, the choice between craniospinal and focal irradiation, and the appropriate radiation dose should be cautiously determined for these patients.
In this review, I have described the characteristics of patients with relapsed MBL, including the observed patterns of relapse, and the most commonly prescribed treatment. Additionally, I have reviewed the re-irradiation studies and the discussed pertinent concerns, to conclusively suggest the RT recommendations for patients with relapsed MBL.
The treatments for relapsed MBL are likely associated with the nature of relapse and subsequent disease course. A UK Children’s Cancer and Leukemia Group study showed a prolonged disease-free interval and an associated likelihood of local recurrence in patients who received upfront craniospinal irradiation (CSI), compared to those who did not receive it. In the non-irradiated group, the application of CSI at relapse was associated with improved survival [15]. In addition, there is an association between relapse patterns and molecular subgroups; this was demonstrated by a study on recurrent MBL conducted in the Hospital for Sick Children. When recurred, the subgroup affiliation remained stable and group 4 tumors recurred significantly later than both group 3 and SHH tumors. The recurrences were predominantly local in SHH tumors and metastatic in group 3 and group 4 tumors. However, treatment with CSI at the time of diagnosis was not significantly associated with the anatomical pattern of recurrence [16].
According to the data on relapse sites enlisted in Table 1, the neuraxial dissemination through cerebrospinal fluid pathways is observed to be predominant. The isolated local relapse in the posterior fossa (PF) and relapse in distant metastatic areas, with or without synchronous PF relapse, develops in approximately 20% and >80% of patients, respectively [91718]. However, the relapse pattern varies with the use of upfront RT. A UK Children’s Cancer and Leukemia Group study reported higher local relapse rates in cases without upfront RT. The local, distant, and combined relapse rates were 16%, 57%, and 27% respectively, in those who underwent upfront RT, while they were 33% each, in the no upfront RT group [15].
For the treatment of patients with relapsed MBL, repeat surgery, chemotherapy and RT can be provided; however, the optimal treatment remains elusive. In cases of relapsed MBL in infants, previously treated with a radiation-sparing regimen, RT including CSI could be a promising choice [14]. However, re-irradiation, is very challenging due to the fear of late complications as most of them have been treated with upfront CSI. Therefore, RT is not the primary salvage treatment modality. Tables 2 and 3 show that only 22% of patients with relapsed MBL were treated with RT. Surgery and HDCT with stem cell rescue were more frequently used compared to focal RT in isolated PF relapses. In cases of upfront CSI, only focal RT was delivered although the patients developed multiple craniospinal seeding, as shown in Table 2 [9], and only 1% of patient was treated with CSI (Table 3). CSI was delivered to only 31% of patients without an upfront RT [15]. Considering more than 80% of relapses developing in the craniospinal axis as showed in Table 1, focal RT is not sufficient to achieve tumor control.
Unlike relapsed MBL, re-irradiation is actively applied for treatment of other pediatric brain tumors, and plays an important role, especially in relapsed ependymoma [192021]. Furthermore, Tsang et al. [22] recommended CSI for patients with local recurrence. In this retrospective cohort study of 22 patients with locally recurrent ependymoma, higher 5-year freedom from progression was associated with re-irradiation with CSI (83.3%) than with focal re-irradiation (15.2%). Therefore, re-irradiation could be a valuable treatment option in patients with relapsed brain tumors despite its consequences. Likewise, in the treatment of relapsed MBL, pediatric oncologists consider re-irradiation, when the clinical benefits outweigh the risk for toxicity.
Table 4 enlists several retrospective studies over the last 10 years regarding re-irradiation in patients with relapsed MBL [2324252627]. The sample size were small (<30), and most patients received CSI as initial treatment. Table 4 shows an overall survival (OS) advantage of re-irradiation as part of a multidisciplinary approach over the historical reports, which showed less than 25% of expected 2-year OS after disease progression [82829]. The radiation damage from re-irradiation seems tolerable. These results appear quite promising, regarding the efficacy and safety of salvage re-irradiation. The most significant observation, as shown in Table 4, is the shift of re-irradiation target from focal areas to the whole craniospinal axis. However, radiation-induced toxicity in patients with relapsed MBL must be considered while opting for re-CSI.
Before the selection of re-irradiation for relapsed MBL patients, several concerns should be considered.
Thirty-eight patients with disease progression after treatment at St. Jude Children’s Research Hospital were analyzed for survival outcomes and toxicities based on re-irradiation [24]. All patients received CSI as initial treatment. Among 14 patients who received re-irradiation, more than half received CSI, and the overall survival rates were prolonged among both standard- and high-risk patients. The 5- and 10-year OS rates were 55%±14% and 33%±16%, respectively in standard-risk patients with additional irradiation, however, those were 46%±14% and 0% for others (p=0.036). Similar improvement was observed in high-risk patients according to irradiation (p=0.003). The most common radiotoxic effect was intra-tumoral hemorrhage, as documented by neuroimaging, and the rate of hemorrhage was similar between the patients irrespective of re-irradiation. The observed rate of hemorrhage was 64.3% (9 of 14) in irradiated patients and it was 62.5% (15 of 24) in the unirradiated patients (p=1.000). Brain necrosis developed in 16 patients, with a higher proportion in re-irradiation group, which were 64% (9 of 14) and 29% (7 of 24) for irradiated and unirradiated patients, respectively (p=0.047). However, the necrosis was transient with CTCAE grade 1 or 2. Therefore, those patients did not receive any additional therapy to treat the subclinical necrosis. Despite the possibility of selection bias in re-irradiation group, the use of irradiation could prolong the survival of these patients with relapsed MBL. Dunkel et al. [30] compared event-free survival (EFS) in patients treated with HDCT and stem cell rescue. The 5-year EFS rates were 60% and 18% in additional RT group and no RT group, respectively (p=0.070). Although the statistical power was limited by the small number of patients, better EFS was observed in patients who received additional RT as part of their retrieval therapy. These retrospective studies indicated better outcomes with additional RT, and thus, re-irradiation should be recommended for patients with relapsed MBL.
Padovani et al. [31] reported the results of a pilot study of re-irradiation and concomitant metronomic temozolomide (TMZ) therapy in patients with focally recurrent MBL. Five patients were focally re-irradiated, concomitantly with TMZ, either alone or as part of a multidrug metronomic regimen. The authors reported complete responses in all five patients; however, in three patients, relapsed MBL lesions progressed outside the radiation field after 28 months of median follow-up. Only one patient relapsed in the re-irradiation field. The authors insisted a possible radiosensitizing effect of concomitant metronomic TMZ with RT, while the recurrence pattern suggested high possibility of delayed multiple seeding, even in patients with only focally relapsed MBL [31]. UK group reported that CSI at relapse was associated with prolonged survival in patients with no initial irradiation [15] and other groups reported similar results. A study from the Hospital for Sick Children reported 14 patients who received focal (supratentorial in six, infratentorial in one, and spine in seven patients) re-irradiation with distant failure being the predominant pattern of recurrence outside second RT volumes [26]. Baroni et al. [27] reported a retrospective cohort study of 24 patients aged ≤18 years, re-irradiated for recurrent MBL. The second course consisted of CSI and focal RT in 15 and 9 patients, respectively, and survival outcomes were significantly better for children with re-CSI, compared to those with only focal re-irradiation. The 3-year post first failure OS rates were 50% and 0%, respectively (p=0.001). No symptomatic intratumoral hemorrhage or radionecrosis were observed, and the survivors were within mild to moderate intellectual disability range. Further, Gupta et al. [25] reported outcomes of salvage re-irradiation in 28 patients with recurrent/progressive MBL. Repeat CSI was delivered to seven (25%) patients. The salvage re-CSI and dose were independent of the previous CSI doses (standard dose of 36 Gy or reduced dose of 23.4 Gy) and relied on the pattern of relapse (leptomeningeal seeding). One patient developed symptomatic radiation necrosis.
Considering the positive tumor cell response to radiation and the propensity for multiple seeding in relapsed MBL, reirradiation including CSI could result in better survival, as validated by consistent results from many retrospective studies [81516262729]. However, the optimal dose, fractionation, and volume of re-irradiation remain undefined, owing to the uncertainty of radiation tolerance for the brain and spinal cord.
Several studies have tried to determine the radiation tolerance of the brain and spinal cord. Veninga et al. [32] evaluated the survival and quality of life of 42 patients who were reirradiated for relapsed primary brain tumors. The median physical doses of the first and second RT were 50 Gy and 46 Gy, respectively, and the median cumulative biological equivalent doses (BED) were 200.4 Gy (α/β=2 Gy) and 115.2 Gy (α/β=10 Gy), respectively. Long-term complications of re-irradiation were evident in three patients, all of whom had a cumulative BED2 of >204 Gy (with α/β=2 Gy). The authors concluded that re-irradiation was feasible for relapsed tumors, which were initially irradiated with a dose within normal brain tolerance levels. Sminia and Mayer [33] reported that radiation-induced normal brain tissue necrosis following conventional re-irradiation of glioma occurred beyond a cumulative equivalent total doses when applied in 2 Gy fractions (EQD2cumulative) around 100 Gy.
The radiation tolerance for the spinal cord has been studied in animal and human models. Ang et al. [34] performed an animal study with rhesus monkeys and delivered two radiation courses to the cervical and upper thoracic spinal cord at 2.2 Gy per fraction. Among 45 monkeys who completed the required observation period of 2–2.5 years post re-irradiation, only four developed myeloparesis. And a simple cumulative dose of 110 Gy given in 2.2 Gy did not harbor morphologically detectable lesions and the recovery of initial radiation dose at 1, 2, and after ≥3 years was 50%, 60%, and 65%–70%, respectively. Nieder et al. [35] concluded that the risk of radiation myelopathy appears small after ≤135.5 Gy2 if the radiation interval is >6 months and the dose of each course is ≤98 Gy2. Thus, the lower risk of radiation myelopathy and higher recovery rate from initial radiation were validated. Therefore, in most re-irradiation studies, the cumulative radiation doses were marginally higher despite a risk of neurocognitive outcomes [23252627].
Approximately 20%–30% of patients with MBL experience tumor relapse, and >80% of relapse demonstrate distant leptomeningeal seeding. However, the application of re-irradiation is very challenging because most patients have been treated with upfront CSI. Therefore, only 20% of patients are re-irradiated with most of them being subjected to focal RT rather than CSI. However, re-irradiation with CSI would result in a better survival outcome than focal RT in selected patients. A prospective multicenter longitudinal study is needed to identify those who may benefit most from re-irradiation and to evaluate the role of repeat CSI.
Fig. 1 shows the recommendation algorithm for re-irradiation of patients with relapsed MBL. If the patients received only focal RT or no RT as initial treatment, salvage CSI will be the first treatment option. Conversely, with initial upfront CSI, the appropriate candidates are selected for re-irradiation. With a focal recurrent site in the brain, salvage RT should be considered. CSI rather than focal RT could be recommended when the patient had gross total resection, initial average-risk patients. And the radiation dose should be selected after the possible cumulative radiation dose to brain or spinal cord. In diffuse recurrence, despite only a few patients being candidates for salvage re-irradiation, CSI is preferred.
In conclusion, re-irradiation is a significant salvage treatment option for patients with relapsed MBL. CSI should be always considered, and the appropriate candidates can be identified through the suggested multidisciplinary approach.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article.
References
1. Packer RJ, Sutton LN, D'Angio G, Evans AE, Schut L. Management of children with primitive neuroectodermal tumors of the posterior fossa/ medulloblastoma. Pediatr Neurosci. 1985-86; 12:272–282. PMID: 3039477.
2. Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a children’s cancer group study. J Clin Oncol. 1999; 17:2127–2136. PMID: 10561268.
3. Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006; 7:813–820. PMID: 17012043.
4. Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006; 24:4202–4208. PMID: 16943538.
5. Carrie C, Grill J, Figarella-Branger D, Bernier V, Padovani L, Habrand JL, et al. Online quality control, hyperfractionated radiotherapy alone and reduced boost volume for standard risk medulloblastoma: long-term results of MSFOP 98. J Clin Oncol. 2009; 27:1879–1883. PMID: 19273707.
6. Lannering B, Rutkowski S, Doz F, Pizer B, Gustafsson G, Navajas A, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012; 30:3187–3193. PMID: 22851561.
7. Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of children’s oncology group trial A9961. Neuro Oncol. 2013; 15:97–103. PMID: 23099653.
8. Bowers DC, Gargan L, Weprin BE, Mulne AF, Elterman RD, Munoz L, et al. Impact of site of tumor recurrence upon survival for children with recurrent or progressive medulloblastoma. J Neurosurg. 2007; 107(1 Suppl):5–10. PMID: 17644914.
9. Sabel M, Fleischhack G, Tippelt S, Gustafsson G, Doz F, Kortmann R, et al. Relapse patterns and outcome after relapse in standard risk medulloblastoma: a report from the HIT-SIOP-PNET4 study. J Neurooncol. 2016; 129:515–524. PMID: 27423645.

10. Koschmann C, Bloom K, Upadhyaya S, Geyer JR, Leary SE. Survival after relapse of medulloblastoma. J Pediatr Hematol Oncol. 2016; 38:269–273. PMID: 26907655.
11. Johnston DL, Keene D, Strother D, Taneva M, Lafay-Cousin L, Fryer C, et al. Survival following tumor recurrence in children with medulloblastoma. J Pediatr Hematol Oncol. 2018; 40:e159–e163. PMID: 29432312.
12. von Bueren AO, von Hoff K, Pietsch T, Gerber NU, Warmuth-Metz M, Deinlein F, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol. 2011; 13:669–679. PMID: 21636711.
13. Dhall G, Grodman H, Ji L, Sands S, Gardner S, Dunkel IJ, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “head start” I and II protocols. Pediatr Blood Cancer. 2008; 50:1169–1175. PMID: 18293379.
14. Muller K, Mynarek M, Zwiener I, Siegler N, Zimmermann M, Christiansen H, et al. Postponed is not canceled: role of craniospinal radiation therapy in the management of recurrent infant medulloblastoma--an experience from the HIT-REZ 1997 & 2005 studies. Int J Radiat Oncol Biol Phys. 2014; 88:1019–1024. PMID: 24661654.
15. Hill RM, Richardson S, Schwalbe EC, Hicks D, Lindsey JC, Crosier S, et al. Time, pattern, and outcome of medulloblastoma relapse and their association with tumour biology at diagnosis and therapy: a multicentre cohort study. Lancet Child Adolesc Health. 2020; 4:865–874. PMID: 33222802.
16. Ramaswamy V, Remke M, Bouffet E, Faria CC, Perreault S, Cho YJ, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013; 14:1200–1207. PMID: 24140199.

17. Warmuth-Metz M, Blashofer S, von Bueren AO, von Hoff K, Bison B, Pohl F, et al. Recurrence in childhood medulloblastoma. J Neurooncol. 2011; 103:705–711. PMID: 21069427.

18. Huybrechts S, Le Teuff G, Tauziede-Espariat A, Rossoni C, Chivet A, Indersie E, et al. Prognostic clinical and biologic features for overall survival after relapse in childhood medulloblastoma. Cancers (Basel). 2020; 13:53. PMID: 33375523.

19. Lobon MJ, Bautista F, Riet F, Dhermain F, Canale S, Dufour C, et al. Re-irradiation of recurrent pediatric ependymoma: modalities and outcomes: a twenty-year survey. Springerplus. 2016; 5:879. PMID: 27386327.

20. Zacharoulis S, Ashley S, Moreno L, Gentet JC, Massimino M, Frappaz D. Treatment and outcome of children with relapsed ependymoma: a multi-institutional retrospective analysis. Childs Nerv Syst. 2010; 26:905–911. PMID: 20039045.

21. Eaton BR, Chowdhry V, Weaver K, Liu L, Ebb D, MacDonald SM, et al. Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiother Oncol. 2015; 116:301–308. PMID: 26243681.

22. Tsang DS, Murray L, Ramaswamy V, Zapotocky M, Tabori U, Bartels U, et al. Craniospinal irradiation as part of re-irradiation for children with recurrent intracranial ependymoma. Neuro Oncol. 2019; 21:547–557. PMID: 30452715.

23. Bakst RL, Dunkel IJ, Gilheeney S, Khakoo Y, Becher O, Souweidane MM, et al. Reirradiation for recurrent medulloblastoma. Cancer. 2011; 117:4977–4982. PMID: 21495027.
24. Wetmore C, Herington D, Lin T, Onar-Thomas A, Gajjar A, Merchant TE. Reirradiation of recurrent medulloblastoma: does clinical benefit outweigh risk for toxicity? Cancer. 2014; 120:3731–3737. PMID: 25080363.

25. Gupta T, Maitre M, Sastri GJ, Krishnatry R, Shirsat N, Epari S, et al. Outcomes of salvage re-irradiation in recurrent medulloblastoma correlate with age at initial diagnosis, primary risk-stratification, and molecular subgrouping. J Neurooncol. 2019; 144:283–291. PMID: 31236820.

26. Tsang DS, Sarhan N, Ramaswamy V, Nobre L, Yee R, Taylor MD, et al. Re-irradiation for children with recurrent medulloblastoma in Toronto, Canada: a 20-year experience. J Neurooncol. 2019; 145:107–114. PMID: 31468270.

27. Baroni LV, Freytes C, Fernandez Ponce N, Oller A, Pinto N, Gonzalez A, et al. Craniospinal irradiation as part of re-irradiation for children with recurrent medulloblastoma. J Neurooncol. 2021; 155:53–61. PMID: 34505229.

28. Grodman H, Wolfe L, Kretschmar C. Outcome of patients with recurrent medulloblastoma or central nervous system germinoma treated with low dose continuous intravenous etoposide along with dose-intensive chemotherapy followed by autologous hematopoietic stem cell rescue. Pediatr Blood Cancer. 2009; 53:33–36. PMID: 19326417.

29. Lefkowitz IB, Packer RJ, Siegel KR, Sutton LN, Schut L, Evans AE. Results of treatment of children with recurrent medulloblastoma/primitive neuroectodermal tumors with lomustine, cisplatin, and vincristine. Cancer. 1990; 65:412–417. PMID: 2153428.

30. Dunkel IJ, Gardner SL, Garvin JH Jr, Goldman S, Shi W, Finlay JL. High-dose carboplatin, thiotepa, and etoposide with autologous stem cell rescue for patients with previously irradiated recurrent medulloblastoma. Neuro Oncol. 2010; 12:297–303. PMID: 20167818.

31. Padovani L, Andre N, Gentet JC, Figarella Branger D, Scavarda D, Verschuur A, et al. Reirradiation and concomitant metronomic temozolomide: an efficient combination for local control in medulloblastoma disease? J Pediatr Hematol Oncol. 2011; 33:600–604. PMID: 22042276.
32. Veninga T, Langendijk HA, Slotman BJ, Rutten EH, van der Kogel AJ, Prick MJ, et al. Reirradiation of primary brain tumours: survival, clinical response and prognostic factors. Radiother Oncol. 2001; 59:127–137. PMID: 11325440.

33. Sminia P, Mayer R. External beam radiotherapy of recurrent glioma: radiation tolerance of the human brain. Cancers (Basel). 2012; 4:379–399. PMID: 24213316.

34. Ang KK, Jiang GL, Feng Y, Stephens LC, Tucker SL, Price RE. Extent and kinetics of recovery of occult spinal cord injury. Int J Radiat Oncol Biol Phys. 2001; 50:1013–1020. PMID: 11429229.

35. Nieder C, Grosu AL, Andratschke NH, Molls M. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys. 2006; 66:1446–1449. PMID: 17084560.

Fig. 1
The recommendation algorithm for radiotherapy in patients with relapsed medulloblastoma. MBL, medulloblastoma; CSI, craniospinal irradiation; RT, radiotherapy.
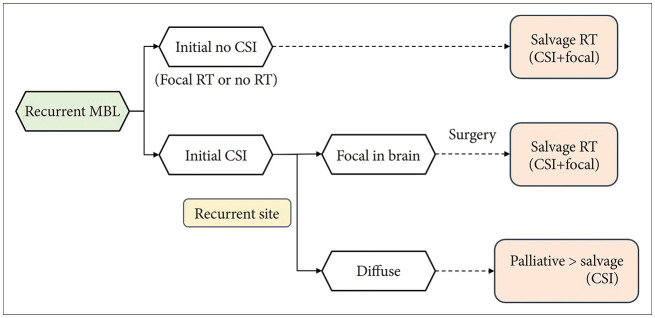
Table 1
The patterns of relapse

| Relapse site | ||
|---|---|---|
| Local | Distant | |
| Sabel et al.* [9] (n=72) | 13 (17) | 59 (83) |
| Warmuth-Metz et al. [17] (n=40) | 8 (20) | 32 (80) |
| Huybrechts et al. [18] (n=48) | 9 (19) | 39 (81) |
Table 2
Treatment for relapsed medulloblastoma from HIT-SIOP-PNET study
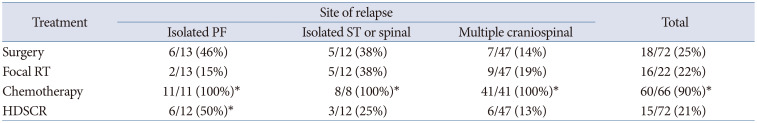
*Including missing data. RT, radiotherapy; HDSCR, high-dose chemotherapy and stem cell rescue; PF, posterior fossa; ST, supratentorial.
Adapted from Sabel et al. J Neurooncol 2016;129:515-524 [9].
Table 3
Treatment for relapsed medulloblastoma according to previous irradiation [15]
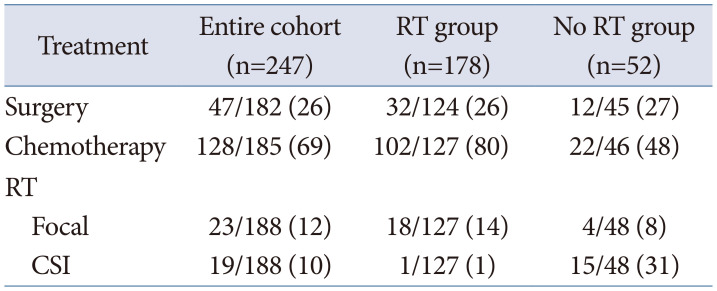
Table 4
Retrospective studies for re-irradiation to the patients with relapsed medulloblastoma
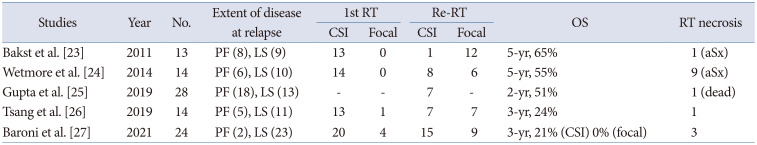
| Studies | Year | No. | Extent of disease at relapse | 1st RT | Re-RT | OS | RT necrosis | ||
|---|---|---|---|---|---|---|---|---|---|
| CSI | Focal | CSI | Focal | ||||||
| Bakst et al. [23] | 2011 | 13 | PF (8), LS (9) | 13 | 0 | 1 | 12 | 5-yr, 65% | 1 (aSx) |
| Wetmore et al. [24] | 2014 | 14 | PF (6), LS (10) | 14 | 0 | 8 | 6 | 5-yr, 55% | 9 (aSx) |
| Gupta et al. [25] | 2019 | 28 | PF (18), LS (13) | - | - | 7 | - | 2-yr, 51% | 1 (dead) |
| Tsang et al. [26] | 2019 | 14 | PF (5), LS (11) | 13 | 1 | 7 | 7 | 3-yr, 24% | 1 |
| Baroni et al. [27] | 2021 | 24 | PF (2), LS (23) | 20 | 4 | 15 | 9 | 3-yr, 21% (CSI) 0% (focal) | 3 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download