INTRODUCTION
Gliomas are the most common primary parenchymal brain tumors in the United States [
1]. They have been histologically diagnosed as the third most common primary tumor of the central nervous system (CNS) in a relatively small portion of Korea [
2]. According to the new World Health Organization (WHO) classification of CNS tumors in 2021 [
3], grade 1 gliomas include 1) diffuse astrocytoma (MYB- or MYBL1-altered) and 2) polymorphous low-grade neuroepithelial tumors in young individuals. Grade 2 and 3 gliomas include 1) astrocytoma (isocitrate dehydrogenase [
IDH]-mutant), 2) oligodendroglioma (
IDH-mutant and 1p/19q-codeleted), and 3) pleomorphic xanthoastrocytoma. Grade 4 gliomas include 1) astrocytoma (
IDH-mutant), 2) glioblastoma (
IDH-wildtype), and 3) diffuse hemispheric glioma (H3G34-mutant). Despite the rarity of gliomas among primary CNS tumors, the disease entity is very dynamic due to its various molecular characteristics, compared with other CNS tumors. The classification of gliomas has been frequently modified due to further advancement of molecular genetic information. The recent WHO classification of CNS tumors was officially published in 2016 as a revised version of the 4th edition of the WHO classification of CNS tumors without full establishment of a concrete diagnostic process because of the rapid advancement of molecular and genetic approaches to diagnose gliomas. Consecutively, the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) was established to enhance the understanding of the molecular pathogenesis of brain tumors, warranting more rapid integration of this information into clinical practice before the official WHO update. The goal of cIMPACT-NOW was to facilitate input and consensus review of novel diagnostically relevant data and determine how advanced molecular and genetic information could be practically incorporated into future CNS tumor classifications [
4]. Although these efforts are ongoing, the practice of managing glioma patients is not globally established as a precise standard protocol because of the different socio-medical environments in individual countries.
In Korea, a working group was appointed by the Korean Society for Neuro-Oncology (KSNO) in February 2018 to establish guidelines for the management of patients with brain tumors. The working group published the KSNO guidelines for adult glioblastoma patients in April 2019 and for grades 2 and 3 glioma patients in October 2019. These guidelines were optimized under unique medical circumstances in Korea. Although the KSNO guidelines were mainly based on the National Comprehensive Cancer Network (NCCN) and European Association of Neuro-Oncology (EANO) guidelines with modifications and changes according to the unique background of Korea, there are several points with discrepancies. In this review, the individual guidelines for glioma by KSNO, NCCN, and EANO will be analyzed and compared with each other. Consequently, this review is expected to be helpful and informative to update KSNO guidelines for glioma patients.
DIAGNOSIS
KSNO guidelines recommend a multidisciplinary approach for treatment planning, if feasible, when the radiological features suggest a glioma. Magnetic resonance imaging (MRI) with contrast enhancement is considered an essential imaging tool for the diagnosis of gliomas. To obtain sufficient tissue for histopathological diagnosis, neurosurgical intervention is mandatory, even if it is for stereotactic biopsy. To achieve maximal safe resection, neuronavigation systems, intraoperative computed tomography (CT) or MRI, intraoperative ultrasonography, intraoperative mapping techniques, and fluorescence-guidance with 5-aminolevulinic acid (ALA) are recommended. Histopathological diagnosis should be officially based on the 2016 WHO classification of CNS tumors [
5]. For the workup of gliomas, codeletion of 1p/19q testing and
IDH1 and
IDH2 mutation tests are essential for molecular diagnosis of gliomas, as well as histopathological features [
678]. The O
6-methylguanine-DNA-methyltrasferase (
MGMT) promoter methylation test is required to predict the response to treatment for all grades of gliomas, especially glioblastoma [
6]. An ATP-dependent helicase (
ATRX) mutation test is also required for the workup of grade 2 gliomas. If the tumor has
IDH-wildtype, the following molecular tests are strongly recommended: 1) epidermal growth factor receptor (
EGFR) amplification, 2) combined whole chromosome 7 gain and whole chromosome 10 loss, and 3) telomerase reverse transcriptase (
TERT) promoter mutation. If the histopathological diagnosis is WHO grade 2 gliomas, patients with the aforementioned molecular features of glioblastoma should be treated on the basis of the guidelines for WHO grade 4 glioblastoma [
8].
The NCCN guidelines are similar to those of KSNO in terms of the diagnostic process. The principles of surgical resection include gross total resection with minimal surgical morbidity using an intraoperative microscope, frameless stereotactic image guidance, preoperative functional MRI and/or diffusion tensor image fiber tracking, awake craniotomy, motor and/or speech mapping, intraoperative MRI, and intraoperative fluorescence-guided surgery with 5-ALA [
9]. For standard histological examination and classification, histological subgrouping of CNS neoplasms is recommended to follow the WHO classification of CNS tumors, and it should be considered an inherently subjective nature of certain aspects of histological interpretation. In addition, the NCCN guidelines state that molecular/genetic characterization does not replace standard histological assessment, but serves as a complementary approach to providing additional diagnostic and prognostic information that often enhances treatment selection. In terms of analytic methods, genome-wide profiling of CpG methylation patterns can be a powerful way to classify brain tumors, including those with equivocal histological features. In accordance with the KSNO guidelines, the molecular features of glioblastoma in lower grade gliomas are also emphasized to have similar clinical outcomes as those of the typical WHO grade 4 glioblastoma and to be treated with standard therapy for grade 4 glioblastoma [
9]. In addition, NCCN panels encourage molecular testing of glioblastomas if a driver mutation, such as
BRAF 600E-activating mutations or neurotrophic tyrosine receptor kinase (
NTRK) fusion, is detected, because it may be reasonable to treat with a target therapy in clinical trials and/or because of its intractable status against conventional treatment. The following molecular markers are recommended for use by neuropathologists to facilitate characterization of gliomas, and/or neuro-oncologists to guide treatment decisions:
IDH1 and
IDH2 mutations, codeletion of 1p and 19q,
MGMT promoter methylation,
ATRX mutation,
TERT promoter mutation, H3F2A mutation, and
BRAF mutation [
9].
The EANO guidelines recommend that treatment decisions in patients with glioma should be made on the basis of tissue diagnosis, including the assessment of molecular markers relevant for diagnosis; therefore, upfront surgery is commonly performed with both diagnostic and therapeutic intent [
10]. To avoid surgical morbidity and adverse outcomes from inadequate biopsy sampling, the EANO guidelines state that the surgical management of patients with glioma should take place in high-volume specialist centers where large numbers of patients are referred to specialist neurosurgeons rather than individual neurosurgeons’ surgical practice [
11]. The EANO guidelines state the importance of tissue diagnosis more concretely than those by KSNO and NCCN; if possible, some tumor tissue should be cryopreserved for molecular assessments that require high-quality DNA and RNA samples. In the same way as the KSNO and NCCN guidelines, the diagnostic process should follow the 2016 WHO classification and subsequent recommendations from cIMPACT-NOW, as the following molecular biomarkers are central to categorizing gliomas in adults:
IDH mutation, 1p/19q codeletion, histone H3K27M mutation, histone H3.3 G34R/V mutation,
TERT promoter mutation,
EGFR amplification, chromosome 7 gain combined with chromosome 10 loss (the +7/–10 signature), and homozygous deletions on 9p21 involving the CDKN2A and CDKN2B gene loci (CDKN2A/B homozygous deletion). Accordingly, glioma classification integrates histological tumor typing and grading, and analyses of molecular markers. Additional recommendations by EANO are as follows: 1) immunohistochemistry for mutant
IDH1 R132H protein and nuclear expression of
ATRX should be performed routinely in the diagnostic assessment of diffuse gliomas; 2) if immunohistochemistry for
IDH1 R132H is negative, sequencing of
IDH1 codon 132 and
IDH2 codon 172 should be conducted in all WHO grade 2 and 3 diffuse astrocytic and oligodendroglial gliomas and in all glioblastomas of patients aged <55 years, to enable integrated diagnoses according to the WHO classification and to guide treatment decisions; 3) 1p/19q codeletion status should be determined in all
IDH-mutant gliomas with retained nuclear expression of
ATRX; 4)
MGMT promoter methylation status should be determined in glioblastomas, notably in elderly or frail patients, to aid in decision-making for the use of temozolomide; 5) CDKN2A/B homozygous deletions should be explored in
IDH-mutant astrocytomas; 6) combined chromosome 7 gain and chromosome 10 loss (+7/–10 signature),
EGFR amplification, and
TERT promoter mutation should be tested in
IDH-wildtype diffuse gliomas lacking microvascular proliferation and necrosis as histological features of WHO grade 4 glioblastoma to allow for a diagnosis of
IDH-wild-type glioblastoma; 7) assessment of H3K27M status should be done in diffuse gliomas involving the midline; and 8)
BRAF V600 mutations might be assessed in
IDH-wild-type diffuse gliomas.
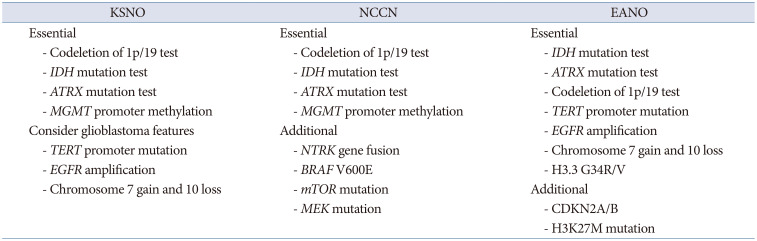
The KSNO and NCCN guidelines commonly recommend the following histological and molecular genetic studies as essential steps for diagnosis and grading of gliomas: 1) codeletion of 1p/19 test, 2)
IDH mutation test, 3)
ATRX mutation test, and 4)
MGMT promoter methylation status (
Table 1). For the determination of therapeutic strategies, the following molecular genetic analyses are also recommended: 1)
TERT promoter mutation, 2)
EGFR amplification, and 3) chromosome 7 gain and 10 loss. NCCN considers the following molecular genetic studies for further target therapy in clinical trials: 1)
NTRK gene fusion, 2)
BRAF V600E, 3) mammalian target of rapamycin mutation, and 4) methyl ethyl ketone mutation. EANO recommends a detailed molecular genetic analysis for glioma diagnosis and grading, such as
IDH mutation test,
ATRX mutation test, codeletion of 1p/19 test,
TERT promoter mutation,
EGFR amplification, chromosome 7 gain and 10 loss, and H3.3 G34R/V (
Table 1).
ADJUVANT TREATMENT for GLIOBLASTOMA
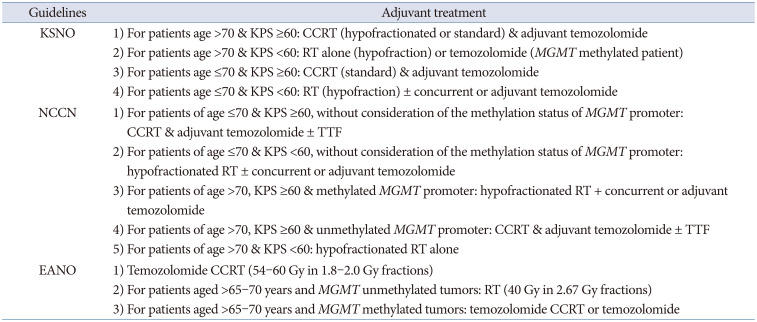
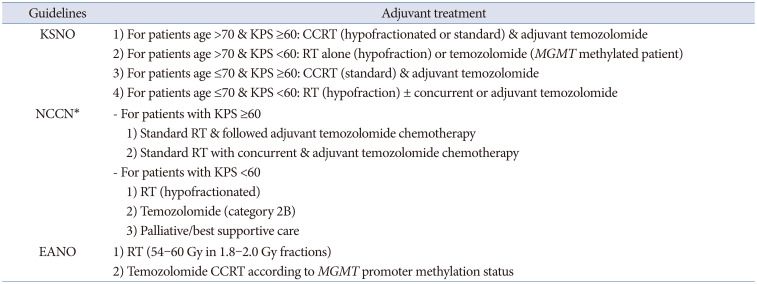
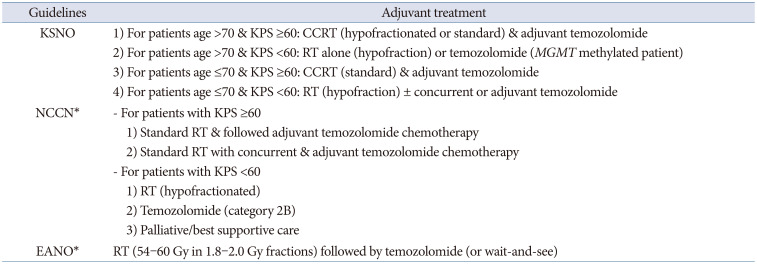
The KSNO guidelines classified glioblastoma patients according to age (≤70 years vs. >70 years) and the Karnofsky Performance Scale (KPS, <60 vs. ≥60) for the application of the protocol. Among glioblastoma patients aged ≤70 years, if the patient has a good performance status (KPS ≥60 or Eastern Cooperative Oncology Group [ECOG] performance score ≤2), concurrent chemoradiotherapy with temozolomide followed by adjuvant temozolomide chemotherapy (Stupp’s protocol) [
12], or standard brain radiotherapy alone should be considered. However, if the patient has a poor performance status (KPS <60 or ECOG performance score ≥3), hypofractionated brain radiotherapy (preferred) ± concurrent or adjuvant temozolomide, temozolomide alone (Level III), or supportive treatment can be considered. In contrast, for glioblastoma patients aged >70 years, if the patient has a good performance status (KPS ≥60 or ECOG performance score ≤2), the following therapeutic options should be considered: hypofractionated brain radiotherapy + concurrent and adjuvant temozolomide, concurrent chemoradiotherapy with temozolomide followed by adjuvant temozolomide chemotherapy, or hypofractionated brain radiotherapy alone. If the patient has a poor performance status (KPS <60 or ECOG performance score ≥3), the following therapeutic options should be considered: hypofractionated brain radiotherapy alone, temozolomide chemotherapy if methylated
MGMT promoter (Level III) is present, or supportive treatment. In poorly performing patients or the elderly, a hypofractionated accelerated course is reasonable with the goal of completing the treatment within 2–3 weeks [
6].
Similarly, the NCCN guidelines classified glioblastoma patients according to age (≤70 years vs. >70 years) and KPS (<60 vs. ≥60) for the application of the protocol, and also considered the methylation status of the
MGMT promoter in patients aged ≤70 years with a good performance scale. The NCCN guidelines preferentially recommend that all glioblastoma patients participate in clinical trials for eligible conditions. Among glioblastoma patients aged ≤70 years with a good performance status (KPS ≥60) and methylated
MGMT promoter, concurrent chemoradiotherapy with temozolomide followed by adjuvant temozolomide chemotherapy ± alternating electrical field therapy or standard brain radiotherapy + concurrent temozolomide and adjuvant lomustine, and temozolomide chemotherapy should be considered. Among them, if the patient has a good performance status (KPS ≥60) with an unmethylated
MGMT promoter, concurrent chemoradiotherapy with temozolomide followed by adjuvant temozolomide chemotherapy ± alternating electrical field therapy, or standard brain radiotherapy alone should be considered. In addition, if the patient has a poor performance status (KPS <60), hypofractionated RT ± concurrent or adjuvant temozolomide should be preferentially considered [
9].
The EANO guidelines recommend Stupp’s regimen as the standard of care for patients with
IDH-wildtype glioblastoma, aged <70 years and with a KPS >70. They also recommend considering the methylation status of the
MGMT promoter when determining the therapeutic option in elderly patients (aged >65–70 years) who are not considered candidates for Stupp’s regimen as follows: 1) radiotherapy (40 Gy in 2.67 Gy fractions) for patients with
MGMT promoter-unmethylated tumors and 2) temozolomide chemoradiotherapy or temozolomide for patients with
MGMT promoter-methylated tumors. The EANO guidelines suggest that temozolomide might only be active in patients with
MGMT promoter-methylated tumors, whereas its activity in patients with
MGMT promoter-unmethylated tumors is probably marginal [
10]. Distinctively, the EANO guidelines recommend temozolomide chemoradiotherapy (54–60 Gy in 1.8–2.0 Gy fractions) without concomitant temozolomide for the potential treatment regimens for WHO grade 4 astrocytoma,
IDH-mutant (cIMPACT-NOW, previously glioblastoma,
IDH-mutant, WHO grade 4) [
10].
The criterion of elderly patients being at least 70 years old was the same in the KSNO and NCCN guidelines. However, the EANO guidelines included ages 65–70 years in their classification of elderly patients. Therefore, there is no concrete definition of old age. Many investigators have reported their data based on old age of >70 years. Although there is uniform consensus for old age (>70 years) among the KSNO working group, a worldwide retrospective multi-institute study can help establish a meaningful age with a prognostic turning point. In principle, the NCCN and EANO guidelines recommend different strategies based on the
MGMT promoter methylation status [
910]. However, the KSNO guidelines were the same as the standard treatment with Stupp’s regimen, regardless of the methylation status of the
MGMT promoter in patients with good performance status (
Table 2). One of the reasons is that the Korean government’s health insurance does not cover alternating electric field therapy. Another difference in the KSNO guidelines from that of NCCN and EANO is the strength of consideration for clinical trials; enrollment for clinical trials is preferentially considered in Western countries. The medical circumstances and environment in Korea are not activated as much as in Western countries because of several cultural and commercial reasons. To overcome these limitations in Korea, co-operation is necessary in neuro-oncology societies.
SALVAGE TREATMENT FOR RECURRENT GLIOBLASTOMA
The KSNO guidelines recommend that the true recurrence of glioblastoma be distinguished from pseudoprogression on MRI within the first 3 months after completion of concurrent chemoradiotherapy with temozolomide. These guidelines state that the following radiologic findings can suggest recurrence of glioblastomas: 1) increase of 25% or more in enhancing lesions despite stable or increasing steroid dose, 2) significant increase of the lesion in the fluid-attenuated inversion recovery (FLAIR) image and T2-weighted image, not attributable to other non-tumor causes, and 3) any new lesions. In addition, if clinical deterioration (not attributable to other non-tumor causes and not due to steroid decrease) occurs simultaneously, true progression must be strongly suggested. When the clinical and radiological features of any grade of glioma, including glioblastoma, is suggested to progress or recur, surgical resection is always recommended, if feasible. The KSNO guidelines recommend the following therapeutic options: 1) systemic chemotherapy (bevacizumab alone, bevacizumab plus irinotecan, daily temozolomide of low dose, lomustine or carmustine, and PCV or procarbazine plus lomustine), and/or 2) reirradiation (especially if there is a long interval since prior radiotherapy and/or if there was a good response to prior radiotherapy), and/or supportive treatment if the patient has poor performance status [
6]. The efficacy of standard-of-care treatment, such as adjuvant temozolomide chemotherapy for recurrent glioblastoma, is suboptimal for salvage purposes. Thus, for eligible patients, clinical trials are highly encouraged [
6].
The NCCN guidelines preferentially recommend clinical trial enrollment for eligible patients. If ineligible, systemic treatments, such as bevacizumab (single or combination with carmustine, lomustine, or temozolomide), temozolomide chemotherapy, lomustine or carmustine, PCV chemotherapy, and regorafenib; reirradiation; alternating electric field therapy; and palliative/best supportive care are considered. If failure or intolerance to the preferred or other recommended regimens occurs, etoposide or platinum-based regimens can also be considered [
9].
The EANO guidelines recommend the selection of treatment modalities based on prior therapy, age, KPS,
MGMT promoter methylation status, and patterns of disease progression. A second surgery can be an option for symptomatic patients with circumscribed relapses diagnosed not earlier than 6 months after the initial surgery. The main systemic treatment options for patients with disease progression include nitrosoureas, temozolomide rechallenge, and bevacizumab (depending on availability). Bevacizumab is not approved for patients with recurrent glioblastoma in the European Union (EU), although it has been approved for this indication in other countries based on the objective response rates of approximately 30% in two uncontrolled phase II trials [
1314]. When available, recruitment into appropriate clinical trials should be considered [
10].
There is no significant difference among the three guidelines, but alternating electric field therapy in the NCCN guidelines is not included in the KSNO and EANO guidelines. Targeted therapy using bevacizumab is mainly recommended in the KSNO and NCCN guidelines, but not in the EANO guidelines because its use in recurrent glioblastoma has been disapproved by the EU. The common recommendation by the three guidelines for recurrent glioblastoma is patient enrollment in clinical trials (
Table 9).
SUMMARIES AND FUTURE DIRECTIONS
In terms of preoperative brain imaging studies, there is no significant difference among the three guidelines. Brain MRI, including T2-weighted, T2-weighted FLAIR sequences and 3D T1-weighted sequences before and after the application of a gadolinium-based contrast agent, is routinely recommended as the diagnostic gold standard for detecting gliomas. CT scans of the brain (with and without contrast) are usually considered for patients who cannot undergo MRI. Magnetic resonance (MR) spectroscopy, MR perfusion imaging, and positron emission tomography (PET)-CT are not routine recommendations but are necessary for specific conditions in clinical practice, such as differentiation of true progression from pseudoprogression.
The common principles of surgical resection of glioma are as follows: gross total resection when appropriate, minimal surgical morbidity, and accurate diagnosis. The following factors should be considered when deciding on surgical resection: age; performance status; feasibility of decreasing the mass effect with surgery; and resectability, including number of lesions, location of lesions, time since last surgery in recurrent patients, and new versus recurrent tumor.
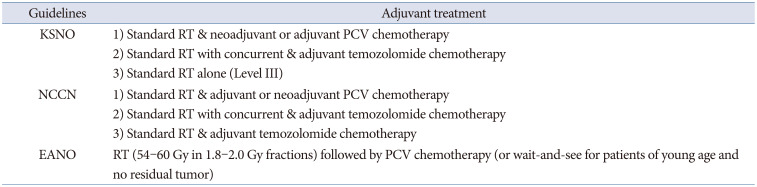
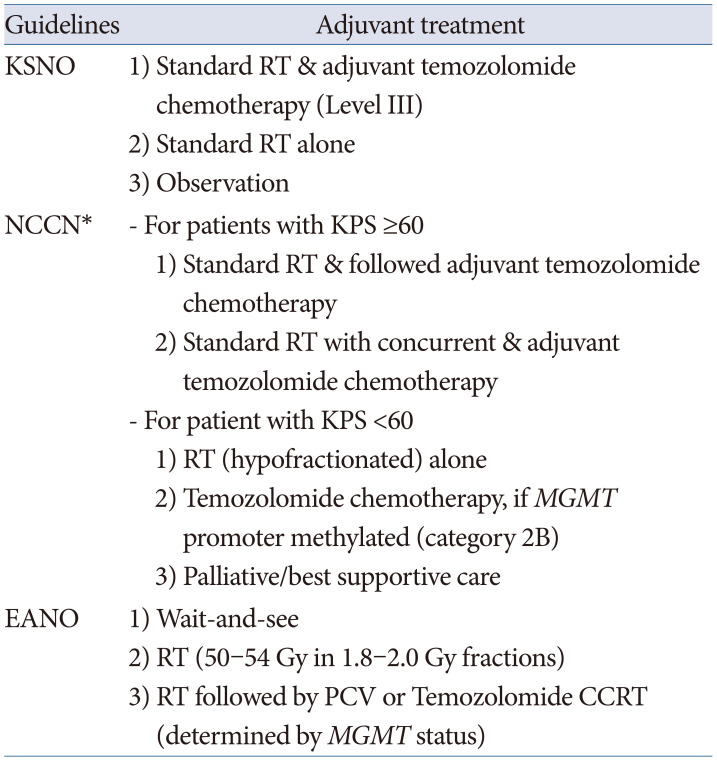
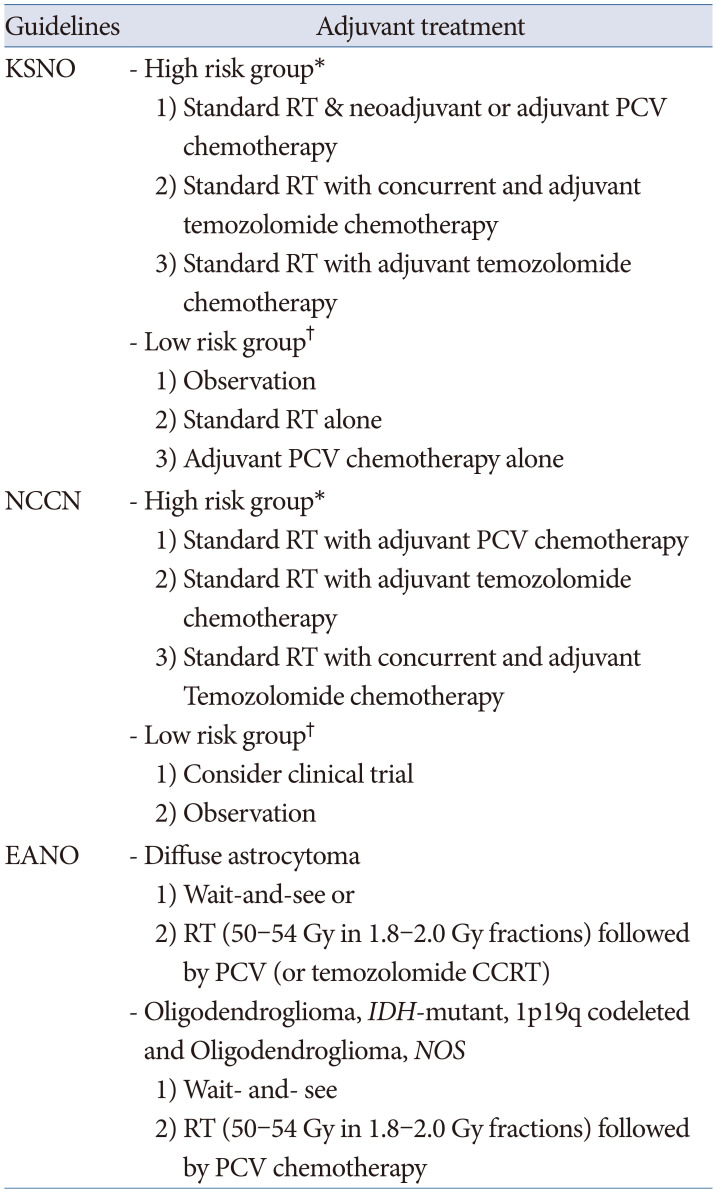
All the guidelines recommend histopathological diagnosis and classification of individual gliomas based on the 2016 WHO classification of CNS tumors. The classification of gliomas by the NCCN guidelines is simpler than that of other guidelines, and the EANO guidelines have a more detailed classification by application of the cIMPACT-NOW recommendation than that of the KSNO and NCCN guidelines. Ultimately, these different histopathological and molecular genetic diagnoses of gliomas should be established for global application based on an updated version of the 2021 WHO classification of CNS tumors.
Adjuvant treatment modalities for individual gliomas are recommended with various optimized protocols according to the different socio-medical environments of individual countries. The EANO guidelines are well established on evidence-based processes, such as detailed systemic reviews and scientific systems for making guidelines. However, the evidence qualities and the strength of recommendation are not the same among the three guidelines, although they have the same protocol. The most recent guidelines for diffuse astrocytoma and oligodendroglial tumors in adults by the American Society of Clinical Oncology (ASCO) and Society for Neuro-Oncology (SNO) on December 13, 2021 have several different recommendations from three guidelines [
15]. For example, the ASCO-SNO guidelines in 2021 recommend deferring initial therapy until radiographic or symptomatic progression in people with oligodendroglioma (
IDH-mutant and 1p19q codeleted) [
15]. The major cause of this discrepancy is the lack of randomized clinical trials due to the rarity of the disease. Therefore, the NCCN and EANO guidelines emphasize patient enrollment in clinical trials immediately after the diagnosis of glioma.
Following the publishing of the guidelines for glioblastoma, WHO grade 2 and 3 gliomas, in adults in 2019 by the KSNO guideline working group, preparations are ongoing to update the guidelines. At the end of 2021, the working group started activating the multidisciplinary committee of the KSNO to update the guidelines. It is expected that many executive members of the disease-specific subcommittee of the multidisciplinary committee of KSNO will participate in the new KSNO guideline working group. For this updated version, a national fund of more than 5 million USD will be granted by the National Cancer Center. In the updated version of the guidelines, a more detailed evidence-based system is essential for application to the practical fields of treating patients. It is necessary for new KSNO guidelines to establish the scientific evidence quality and strength of recommendations more systemically.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download