Abstract
Multiple primary tumors at adjacent site are rare. We report a rare case of coincidentally found nasopharyngeal cancer and ventral foramen magnum meningioma. The 68-year-old male patient presented with a year history of ataxia. Radiological examination revealed lesions in the nasopharyngeal space and ventral foramen magnum. A needle aspiration biopsy for nasopharyngeal space and surgical removal for foramen magnum lesion were performed. The pathological diagnoses were nasopharyngeal cancer and meningioma, respectively. The concomitant occurrence of these two tumors is very rare and there is no known association between these two tumors. We report a case of ventral foramen magnum meningioma simultaneously present with nasopharyngeal carcinoma.
Simultaneous occurrence of multiple brain tumors is usually thought of in patients with genetic disorders such as neurofibromatosis, von Hippel Lindau’s disease, tuberous sclerosis, or those who have received radiation therapy [12]. Multiple tumors at adjacent sites found in patients who do not have these factors are rare.
We present a case of a 68-year-old male patient who presented with gait disturbance and was finally diagnosed with adjacent site foramen magnum meningioma and nasopharyngeal carcinoma.
A 68-year-old male patient was admitted to our hospital with a year history of ataxia. He had difficulties in balance, walking, speaking, and swallowing.
The MRI revealed the dura based well enhancing mass compressing brainstem severely in the ventral foramen magnum suggesting meningioma and another heterogenous mass in the posterior nasopharyngeal wall with invasion into the prevertebral muscles, clivus and extrusion into the sphenoid sinus suggesting nasopharyngeal cancer (Fig. 1). Ultrasonography-guided core needle biopsy was performed on the neck lymph node. On pathologic examination, poorly differentiated basaloid cellular nests in fibrotic stroma (Fig. 2A) and diffusely positive reaction on p63 stain (Fig. 2B) was revealed confirming nasopharyngeal carcinoma.
For the treatment of foramen magnum meningioma, the surgical removal via the far lateral approach was performed. We used medial transposition of vertebral artery and linear dural incision lateral to vertebral artery. The occipital condyle and C1 lateral mass was drilled partially to expose the dura (Fig. 3A). The dural attachment of the tumor in the ventral portion of the foramen magnum and the clivus was exposed from the anterior side of the vertebral artery (Fig. 4A and D). Internal bulking was repeated while detaching the tumor from the origin dura. During the operation, cranial nerve (CN) V, VII, VIII and lower CNs were observed and preserved (Fig. 4B). After the tumor was sufficiently removed, the tumor in the area where the lower CNs responded to the neurostimulator was left (Fig. 4C) as seen on postoperative MRI (Fig. 3B-D). On pathological examination, meningothelial cellular proliferation and hyalinized vessels with occasional microcalcifications (Fig. 2C) and diffusely positive reaction on epithelial membrane antigen stain (Fig. 2D) was shown confirming the meningothelial meningioma.
After surgery, the patient received concurrent chemoradiation therapy using 5-fluorouracil and cisplatin, and remnant meningioma was also included in the range of radiation therapy. At the last follow-up, the patient is stable at 11 months post-surgery.
Nasopharyngeal carcinoma is an epithelial carcinoma arising from nasopharyngeal mucosa. It is known that the 3-year survival rate is 68%–94% and the 5-year survival rate is 78%–86% [34].
Epstein-Barr virus (EBV) infection is known as a risk factor [5]. The loci identified as genetic susceptibility genes conferring nasopharyngeal carcinoma risk include HLA genes residing at the MHC region on chromosome 6p21, TNFRSF19 on 13q12, MECOM on 3q26, CDKN2A/2B on 9p21, CLPTM1L/TERT on 5p15 [67]. Loss-of-function mutations in the NF-kB–negative regulators, CDKN2A/CDKN2B, CCND1 amplification, TP53, and PI3K/MAPK signaling pathways have also been studied as risk factors [68].
As a treatment method, chemotherapy and radiation therapy are performed. In this case, radiation therapy and chemotherapy using 5-fluorouracil and cisplatin were also performed.
Foramen magnum meningioma is a rare tumor accounting for 0.3%–3.2% of all meningiomas [9]. It is defined as a meningioma occurring between the inferior third of the clivus and the superior edge of the C2 body. In other words, it can be defined as a meningioma arising between the jugular tubercle and the C2 laminae and posteriorly between the anterior edge of the squamous occipital bone and the C2 spinous process [1011]. Most are intradural tumors, and in rare cases, up to 10% may have an extradural or intra-extra dural location [11]. According to Lariboisiere hospital’s classification system, this case could be classified as an anteriorly located tumor on both side of the vertebral artery [1012].
Treatment options include close observation and regular check-up, radiosurgery, and surgical removal. Surgical removal is the gold standard of treatment, but it is not easy due to the surrounding important neurovascular structures. Therefore, the treatment option should be set in consideration of the patient’s age, the patient’s condition including comorbidity, neurological abnormalities, and the degree of brainstem compression. If the surgical removal is required, it is difficult to decide which approach to choose. Various surgical approaches have been tried, and the most representative surgical approaches are far lateral approach, extreme lateral approach, anterolateral approach, posterior approach, and extreme-lateral infrajugular transcondylar-transtubercular exposure (ELITE) [1314]. In the case of the above patient, there were symptoms of gait disturbance and weakness in the extremities, and the brainstem was severely compressed, so surgical resection was selected. Structures that should be paid attention to in treating lesions in the foramen magnum include cerebellum, medulla oblongata, lower CN IX–XII, rostral aspect of the spinal cord, upper CNs (C1 and C2), vertebral artery, posterior inferior cerebellar artery (PICA), and anterior and posterior spinal arteries. In particular, preservation of the lower CNs is important to lower surgical morbidity. CN IX, X, and XI emerge from the rootlets of the postolivary sulcus and run into the dorsal of the veterbral artery [15]. CN XII also exits the rootlets of the preolivary sulcus and runs behind the veterbral artery [15]. Access to the ventral side of the veterbral artery allows access to the ventral foramen magnum without touching the lower CNs. Therefore, understanding and approaching the veterbral artery is a very important factor in the operation of foramen magnum meningioma. Since the vertebral artery and surrounding bony structures may have variations including ponticulus posticus, it is important to understand its positional relationship with the C1 transverse foramen, C2 lamina before surgery [16]. In addition, the possibility of extradural origin of PICA should be considered [1718]. There was no abnormal finding in the vertebral artery in this patient. When transposing the vertebral artery, partial removal of the occipitocervical joint is performed. It has been studied that removing about 1/3 to 1/2 does not cause instability problems [19]. In this case, less than 1/3 of the condyle was removed and there was no problem with instability (Fig. 3A).
Molecular alterations found to be associated with meningioma include NF2, AKT1, PI3KCA, TRAF7, TERT promoter, and CDKN2A/B [20]. The genetic association between meningioma and nasopharyngeal carcinoma has not been established. Multiple primary tumors of nasopharyngeal carcinoma and foramen magnum meningiomas are rarely reported. An age-standardized incidence rate is 3.0 per 100,000 in China, and 0.4 per 100,000 in white populations [21]. Its incidence rate has steadily decreased since the 1980s [22]. On the other hand, the incidence of meningiomas reported 14.3% to 19.0% of all intracranial tumors and foramen magnum meningiomas account for only 0.3%–3.2% of all meningiomas [923].
According to one paper about review of multiple primary tumors in the brain, they classified tumors of multicentric origin into anatomical tumors as follows: (a) multiple tumors of a single organ, (b) tumors of bilateral and symmetrically developing organs, (c) tumors of a system of organs, (d) tumors of functionally related organs, and (e) tumors of entirely different and unrelated organs [24]. This case belongs to group (e), which is mutually independent in distant organs.
Studies on the causes of multiple primary tumors in distant organ have not been conducted. One case report said the development of meningioma after the use of radiation for the treatment of nasopharyngeal carcinoma [25]. Moreover, in one study, nasopharyngeal cancer was discovered incidentally during follow-up of a patient with meningioma [26]. Unlike these two cases, it is not known whether the multiple primary tumors mentioned in this paper were discovered by chance or caused by a specific mechanism because the precise pathophysiology of a unique synchronous occurrence is not clearly known. Further research will give a clearer understanding of the synchronous relationship.
Multiple tumors are rare in patients with neither hereditary disease nor medical history, and choice of the treatment option is not easy. The surgeon should be aware of the possibility of coincidental presentation of the nasopharyngeal cancer and foramen magnum meningioma.
Notes
Ethics Statement: This study was conducted under ethical standards. This study was approved by the Institutional Review Board (IRB) of Kangbuk Samsung Hospital (2021-02-029), which waived the requirement to obtain informed consent because all of the data were anonymized under confidentiality guidelines.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Spallone A, Santoro A, Palatinsky E, Giunta F. Intracranial meningiomas associated with glial tumours: a review based on 54 selected literature cases from the literature and 3 additional personal cases. Acta Neurochir (Wien). 1991; 110:133–139. PMID: 1927605.
2. Munjal S, Kumar J, Jain S, Mehta VS. Glioma simultaneously present with adjacent meningioma: case report and literature review. Asian J Neurosurg. 2019; 14:272–274. PMID: 30937052.
3. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009; 27:242–249. PMID: 19064973.
4. Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J Cancer. 2019; 145:295–305. PMID: 30613964.

5. Young LS, Dawson CW. Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer. 2014; 33:581–590. PMID: 25418193.
6. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019; 394:64–80. PMID: 31178151.
7. Cui Q, Feng QS, Mo HY, Sun J, Xia YF, Zhang H, et al. An extended genome-wide association study identifies novel susceptibility loci for nasopharyngeal carcinoma. Hum Mol Genet. 2016; 25:3626–3634. PMID: 27436580.
8. Zhang L, MacIsaac KD, Zhou T, Huang PY, Xin C, Dobson JR, et al. Genomic analysis of nasopharyngeal carcinoma reveals TME-based subtypes. Mol Cancer Res. 2017; 15:1722–1732. PMID: 28851814.
9. Arnautović KI, Al-Mefty O, Husain M. Ventral foramen magnum meninigiomas. J Neurosurg. 2000; 92:71–80. PMID: 10616061.
10. Bruneau M, George B. Foramen magnum meningiomas: detailed surgical approaches and technical aspects at Lariboisière Hospital and review of the literature. Neurosurg Rev. 2008; 31:19–32. discussion 32-3. PMID: 17882459.
11. George B, Lot G, Boissonnet H. Meningioma of the foramen magnum: a series of 40 cases. Surg Neurol. 1997; 47:371–379. PMID: 9122842.
12. Bruneau M, George B. Classification system of foramen magnum meningiomas. J Craniovertebr Junction Spine. 2010; 1:10–17. PMID: 20890409.
13. Wanibuchi M, Fukushima T, Zenga F, Friedman AH. Simple identification of the third segment of the extracranial vertebral artery by extreme lateral inferior transcondylar-transtubercular exposure (ELITE). Acta Neurochir (Wien). 2009; 151:1499–1503. PMID: 19657583.
14. Wen HT, Rhoton AL Jr, Katsuta T, de Oliveira E. Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far-lateral approach. J Neurosurg. 1997; 87:555–585. PMID: 9322846.
15. Rhoton AL Jr. The cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach. Neurosurgery. 2000; 47:S93–S129. PMID: 10983306.
16. Tambawala SS, Karjodkar FR, Sansare K, Motghare D, Mishra I, Gaikwad S, et al. Prevalence of ponticulus posticus on lateral cephalometric radiographs, its association with cervicogenic headache and a review of literature. World Neurosurg. 2017; 103:566–575. PMID: 28427978.
17. Magklara EP, Pantelia ET, Solia E, Panagouli E, Piagkou M, Mazarakis A, et al. Vertebral artery variations revised: origin, course, branches and embryonic development. Folia Morphol (Warsz). 2021; 80:1–12. PMID: 32073130.
18. Li T, Yin YH, Qiao GY, Wang HW, Yu XG. Three-dimensional evaluation and classification of the anatomy variations of vertebral artery at the craniovertebral junction in 120 patients of basilar invagination and atlas occipitalization. Oper Neurosurg (Hagerstown). 2019; 17:594–602. PMID: 31127851.
19. Mazur MD, Couldwell WT, Cutler A, Shah LM, Brodke DS, Bachus K, et al. Occipitocervical instability after far-lateral transcondylar surgery: a biomechanical analysis. Neurosurgery. 2017; 80:140–145. PMID: 28362894.
20. Birzu C, Peyre M, Sahm F. Molecular alterations in meningioma: prognostic and therapeutic perspectives. Curr Opin Oncol. 2020; 32:613–622. PMID: 32890025.
21. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.
22. Lee AW, Foo W, Mang O, Sze WM, Chappell R, Lau WH, et al. Changing epidemiology of nasopharyngeal carcinoma in Hong Kong over a 20-year period (1980-99): an encouraging reduction in both incidence and mortality. Int J Cancer. 2003; 103:680–685. PMID: 12494479.
23. Wara WM, Sheline GE, Newman H, Townsend JJ, Boldrey EB. Radiation therapy of meningiomas. Am J Roentgenol Radium Ther Nucl Med. 1975; 123:453–458.
24. Courville CB. Multiple primary tumors of the brain: review of the literature and report of twenty-one cases. Am J Cancer. 1936; 26:703–731.
25. Stein ME, Drumea K, Bernstein Z, Daoud C, Zarour M, Kuten A. Advanced nasopharyngeal carcinoma and radiation-induced meningioma in dizygotic twins--a rare case report. Acta Oncol. 1996; 35:761–762. PMID: 8938229.
26. Olivero WC, Lister JR, Elwood PW. The natural history and growth rate of asymptomatic meningiomas: a review of 60 patients. J Neurosurg. 1995; 83:222–224. PMID: 7616265.
Fig. 1
Preoperative axial (A), coronal (B), and sagittal (C) gadolinium-enhanced T1 weighted MRI scans. A 4.2×2.3×3-cm sized well enhancing mass at retroclival area is compressing medulla and shows bilateral V4 segments encasements. Another heterogeneous enhancing mass in the posterior nasopharyngeal wall with invasion into the prevertebral muscle and clivus is observed.

Fig. 2
Photomicrograph of nasopharyngeal mass (A and B) and an excised specimen of foramen magnum mass (C and D). On pathological examination, poorly differentiated basaloid cellular nests in fibrotic stroma (A; H&E, ×20) and diffusely positive reaction on p63 stain (B, ×20) were observed confirming nasopharyngeal carcinoma. Meningothelial cellular proliferation and hyalinized vessels with occasional microcalcifications were noted (C; H&E, ×20). EMA stain (×20) shows diffusely positive reactionsuggestive of meningothelial meningioma (D).

Fig. 3
Postoperative axial CT (A), gadolinium-enhanced T1 weighted axial (B), coronal (C) and sagittal (D) MRI scans. A: CT image shows the patient’s bone status after C1 partial laminectomy and partial condylectomy. B-D: Postoperative MRI scans show subtotal removal of the foramen magnum mass with residual tumor in the area where the lower cranial nerves responded to the neurostimulator.
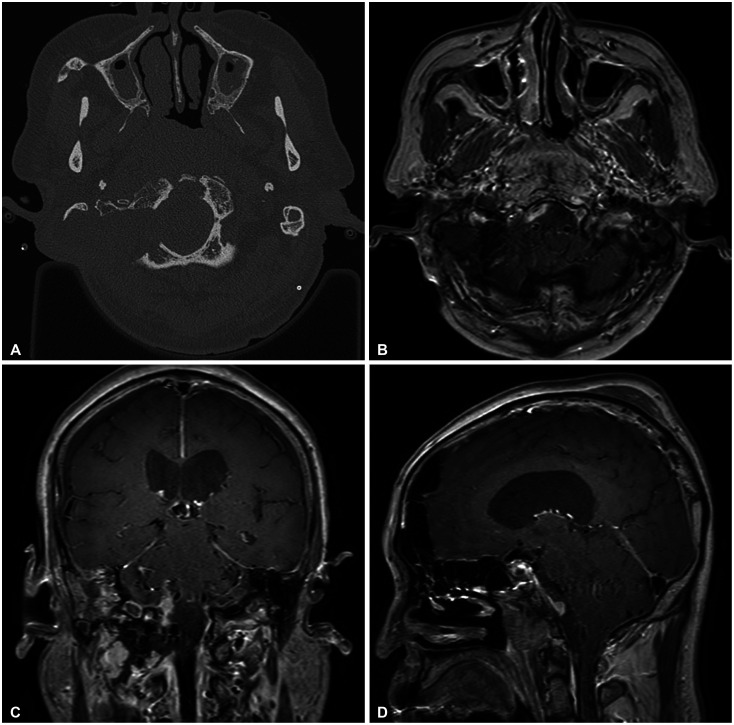
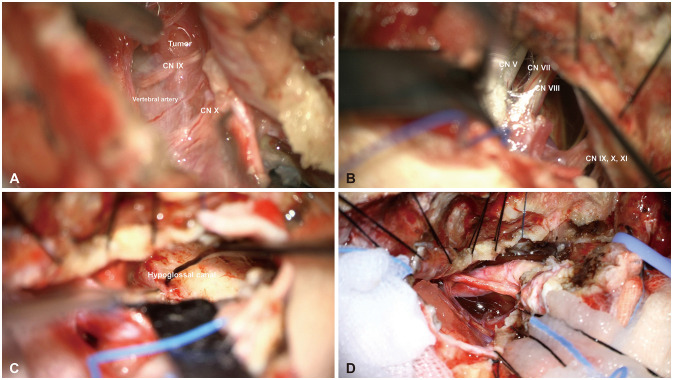
Fig. 4
Intraoperative photographs. A: Preremoval photograph showing the dural attachment of the tumor in the ventral portion of the foramen magnum and clivus from the anterior side of the vertebral artery. B: Surgical image shows cranial nerve (CN) VII, VIII, IX, X, XI remain intact after internal bulking was repeated while detaching the tumor from the dura. C: After sufficiently removing the tumor, the tumor in the lower CN area that responds to the neurostimulator was left behind. D: Postremoval photograph revealing the preserved vertebral artery and lower CNs.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download