Abstract
A natural course of asymptomatic neuroepithelial cysts (NECs) is poorly understood due to its rarity. Herein we report a 23-year-old female patient of an asymptomatic NEC which grew in size from 1 cm to 5 cm and caused progressive symptoms seven years after its incidental finding. Partial resection of the cyst was performed for decompression and pathological examination and effectively achieved symptoms alleviation and regression of the cyst. Our case showed the importance of regular follow-up because NECs may show symptomatic change even in the late phase.
Neuroepithelial cysts (NECs) are relatively rare intracranial benign lesions [1]. There are sporadic case reports of NEC in the literature, but only symptomatic cases treated in the acute phase or asymptomatic cadaver cases are available. Therefore, predicting the prognosis of an asymptomatic NEC is difficult. Herein we report a case of an asymptomatic NEC which grew in size and caused progressive symptoms in the long term.
A 23-year-old woman with complaints of progressive weakness of the right arm spanning over six months visited our outpatient clinic. She had a history of transient diplopia at the age of 16 and underwent MRI which showed a small intra-axial cystic lesion with a single septum in the left frontal lobe (Fig. 1A and B). Given that the cyst was only 1 cm in diameter and apparently irresponsible for the transient diplopia, no follow-up was performed. Seven years later, she presented with mild right hemiparesis. MRI demonstrated that the cyst had grown to 5 cm in diameter (Fig. 1C and D). The content of the cyst showed low intensity on T1-weighted images and high intensity on T2-weighted images. These findings were apparently similar to cerebrospinal fluid; however, region of interest analysis of the content of the cyst showed slightly lower intensity on T1-weighted images compared with the ventricle (ratio, 0.96). No flow voids and no gadolinium-enhanced lesions were identified. The differential diagnoses were NEC, arachnoid cyst, dermoid cyst, choroid plexus cyst, etc. Among these, NEC was the most likely diagnosis given that the cyst existed intra-axially. As she finally presented intracranial hypertension with headache and papilledema, surgical treatment was planned.
Under general anesthesia, a small craniotomy was made in the left forehead (Fig. 2). After 2-cm corticotomy, a daughter cyst was identified. A 1-cm-square piece of the cyst wall was excised and submitted for pathological examination. The septum was easily identified through the daughter cyst and resected along with its associated feeding vessels and submitted for pathological examination. The containing fluid of the cyst was transparent like cerebrospinal fluid and aspirated for decompression purposes, and subsequently, the cavity was refilled with normal saline. The cavity was left open to the subdural space without shunt catheter placement. The protein concentration level of the fluid was 23 mg/dL, which was within normal limits.
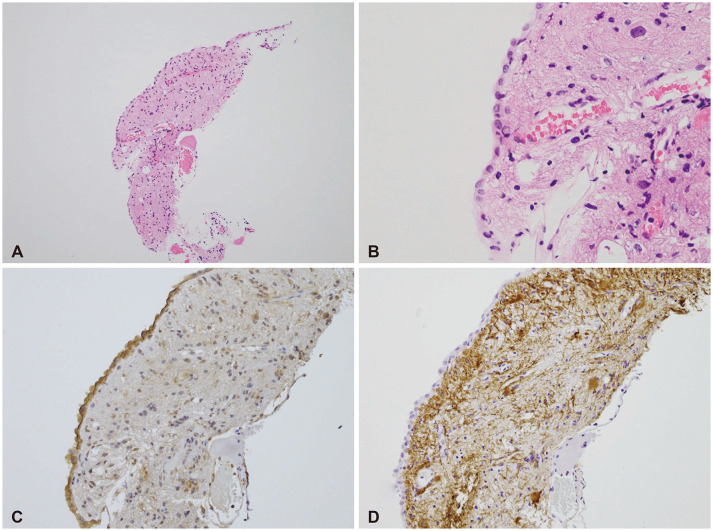
On the pathological examination, the surface of the cyst wall was made of an epithelial layer with cuboidal cells (Fig. 3). The epithelial lining was resting upon connective tissue and no glial layer was identified. Overt cilia were not observed on the surface of the epithelial lining. Several vessels were identified among the connective tissue. There was no infiltration of inflammatory cells. Immunostaining showed that the specimen was positive for epithelial membrane antigen and negative for glial fibrillary acidic protein. These findings were consistent with the diagnosis of NEC.
The symptoms improved immediately after surgery and never recurred. MRI during follow-up demonstrated a gradual reduction of the volume of the cyst (Fig. 1E-H). The cyst was 3 cm in diameter 18 months after surgery.
We presented a case of NEC, which increased in size over a long period of time and caused symptoms and was successfully treated by partial resection. Due to the limited case reports of symptomatic or asymptomatic autopsy ones, the natural history of asymptomatic NEC remains unknown. Previous reports suggest that NECs grow due to increased secretion from epithelial cells rather than from a neoplastic growth [2]; however, our patient had no apparent history of trauma or intracranial inflammatory disease that could have accelerated cyst secretion. Moreover, the evidence of secretion like the presence of cilia was not confirmed pathologically, although this might be because of artifacts in the preparation of the slides. One possible explanation is that local tiny intra-axial inflammation existed and caused extremely slow secretion, which eventually resulted in symptomatic change over a long period of time. Otherwise, ectopic choroid plexus might have existed in the cyst and caused secretion [3]. Unfortunately, the specimen was too small to provide a conclusive explanation for these hypotheses. Another possibility is that the check valve system, one-way inflow into the cyst, affected the cyst enlargement [4].
There is no consensus on the appropriate treatment of NECs. Some authors reported recurrence of NECs and therefore recommended total cyst removal to prevent secretion from the wall of the cyst [56]. However, it is often difficult because of the risk of damaging the surrounding cerebral structures. Since the cyst was located in the premotor gyrus, we chose partial resection considering the risk of motor dysfunction. Shunt catheter placement was an option, which we did not use because of the risk of infection. Chronological regression of the NEC without total cyst removal indicates that resection of the feeding vessels may contribute to decreasing cyst secretion. Several reports support this hypothesis: NECs often have feeding vessels on the wall or septum of the cyst, and these vessels are thought to richly vascularize the epithelial cells to produce secretion [1]. More cases need to be studied to find the risk factors of recurrence and for the consideration of an appropriate treatment.
NEC is the collective name for ependymal and glioependymal cysts [2]. To differentiate these, pathological examinations are essential: ependymal cysts have connective tissue and an epithelial lining which is a single layer of cuboidal epithelial cells, while glioependymal cysts have a glial layer between the connective tissue and epithelial lining [12]. Given that the specimen of the present case did not show a glial layer, we considered that the proper diagnosis was ependymal cyst. However, it should be taken into account that the glial layer could have existed in some parts of the cyst which were not included in the small available specimen. For an accurate diagnosis, wider resection avoiding functional lesions may be better.
We estimated that the chances would be small that an asymptomatic tiny NEC in an adult would grow in size, and did not carry out follow-up. However, this case showed that an asymptomatic small NEC can grow to cause hemiparesis and intracranial hypertension over seven years. We suggest that every intracranial benign cyst, even if asymptomatic, should be followed-up and watched for enlargement and symptomatic change.
Notes
Author Contributions:
Conceptualization: Takeshi Matsuo.
Formal analysis: Ryoko Niwa.
Investigation: Ryoko Niwa.
Methodology: Ryoko Niwa.
Project administration: Ryoko Niwa.
Supervision: Takeshi Matsuo.
Visualization: Takashi Komori.
Writing—original draft: Ryoko Niwa.
Writing—review & editing: Isoo Ayako, Takashi Komori, Takeshi Matsuo.
Ethics Statement
The institutional review board (IRB) exempted informed consent due to its retrospective nature and minimal risk for harm to the patient, and this report was conducted according to the guidelines of the Declaration of Helsinki for biomedical research.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article.
References
1. Robles LA, Paez JM, Ayala D, Boleaga-Duran B. Intracranial glioependymal (neuroglial) cysts: a systematic review. Acta Neurochir (Wien). 2018; 160:1439–1449. PMID: 29802560.

2. Morigaki R, Shinno K, Pooh KH, Nakagawa Y. Giant glioependymal cyst in an infant. J Neurosurg Pediatr. 2011; 7:175–178. PMID: 21284464.

3. Azzam NI, Timperley WR. Intracerebral cyst due to ectopic choroid plexus: case report. J Neurosurg. 1981; 55:651–653. PMID: 7277015.
4. Asamoto S, Fukui Y, Nishiyama M, Ishikawa M, Fujita N, Nakamura S, et al. Diagnosis and surgical strategy for sacral meningeal cysts with check-valve mechanism: technical note. Acta Neurochir (Wien). 2013; 155:309–313. PMID: 23160631.

5. Frazier J, Garonzik I, Tihan T, Olivi A. Recurrent glioependymal cyst of the posterior fossa: an unusual entity containing mixed glial elements. Case report. J Neurooncol. 2004; 68:13–17. PMID: 15174516.

6. Zheng SP, Ju Y, You C. Glioependymal cyst in children: a case report. Clin Neurol Neurosurg. 2013; 115:2288–2290. PMID: 23962758.

Fig. 1
Preoperative and postoperative MRI showing the growth and regression of a cyst. A and B: A sagittal (A) and axial (B) slice of a T1-weighted and T2-weighted image at the age of 16 years showing a small intra-axial cystic lesion with a septum in the frontal lobe. C and D: Preoperative sagittal (C) and axial (D) slice of a T1-weighted and T2-weighted image demonstrating an enlarged cystic lesion compressing the surrounding brain tissue. E-H: Postoperative T1-weighted sagittal image and T2-weighted axial image at 3 months (E and F) and 18 months (G and H) after surgery showing gradual regression of the cyst.
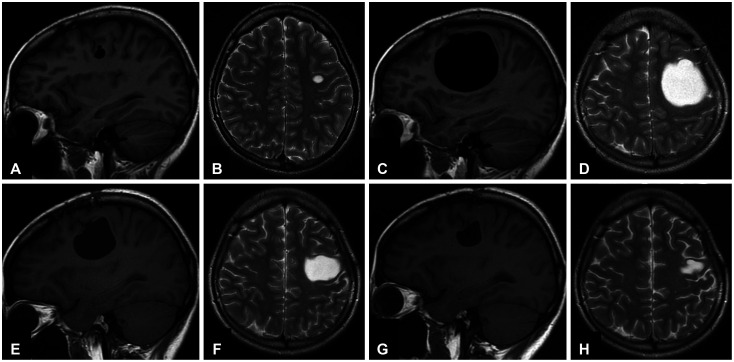
Fig. 2
Operative information and intraoperative findings. A: Three-dimensional reconstructed CT image presenting the craniotomy site. B: An arrow on a sagittal slice of MRI explains the direction of the surgical pathway. C: Intraoperative view under a microscope after a corticotomy showing the septum (arrowhead) and its feeding vessel (asterisk).
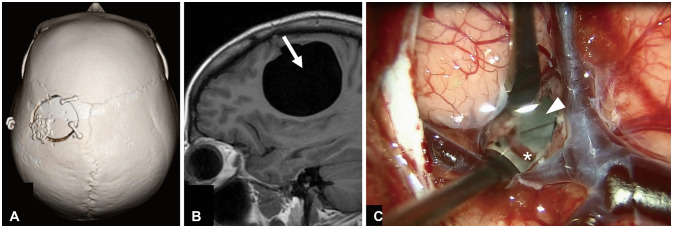
Fig. 3
Operative information and intraoperative findings. A and B: Low power view (A) and high power view (B) of surgical specimen with Hematoxylin-Eosin staining demonstrating a line of cuboidal cells, connective tissue, and vessels. C: Positive immunostaining for epithelial membrane antigen of the epithelial lining. D: Negative immunostaining for glial fibrillary acidic protein indicating the absence of a glial layer.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download