Abstract
Acoustic neuromas are the most common lesion in the cerebellopontine angle. The authors report a unique case of acoustic schwannoma, presenting in middle cranial fossa masquerading as meningioma in a 24-year-old man, presenting with headache and focal seizures. Contrast-enhanced MRI of the brain revealed a mass lesion of the right middle cranial fossa consistent with features of meningioma. Intraoperatively a well-defined tumor with attachment to anterior petrous bone was excised. In the immediate postoperative period, the patient developed right-sided hearing loss, which was proven to be retrochoclear hearing loss on brainstem evoked response audiometry. Histopathology findings were consistent with benign schwannoma. Acoustic schwannoma originating in an unusual location middle cranial fossa is a plausible explanation of such unusual occurrence. Such a case has never been reported in the literature.
Schwannomas constitute 8% of the intracranial tumors. They afflict cranial nerves (CNs) V, VII, VIII, IX, and X in decreasing frequency of occurrence [1]. Schwannomas originate from the transition zone of central nervous glial cells and peripheral nervous Schwann cells known as the Obersteiner–Reidlich zone [2]. Classically, vestibulocochlear schwannoma arises from the inferior division of the vestibular nerve in the internal acoustic canal [3].
We present an unusual case of a 24-year-old male diagnosed with middle cranial fossa meningioma and operated on. He developed hearing loss in the immediate postoperative period. Contrary to preoperative diagnosis, histopathology revealed the tumor to be benign schwannoma. Retrochoclear hearing loss was diagnosed upon audiometry assessment (pure tone audiometery [PTA] and brainstem evoked response audiometery [BERA]). As per the authors’ best knowledge, there have been no similar occurrences reported in the literature. The plausible explanation is schwannoma of CN VIII occurring in the atypical location of the middle cranial fossa.
A 24-year-old male presented with progressively increasing headaches for three months and one episode of focal seizures of the left side not associated with aura. He had no complaints of hearing loss or tinnitus. Clinical examination, including CN examination and gait, was unremarkable. No features of neurofibromatosis were present. Brain MRI (Fig. 1) revealed a heterogeneously enhancing dural-based extra-axial mass lesion in the middle cranial fossa in the temporal lobe region, measuring 25×25×21 mm (craniocaudal×anteroposterior×trans verse). The mass was iso-intense on T2 and T1W images with mild peri-lesional edema. Small areas of necrosis were observed within the lesion.
A provisional diagnosis of meningioma was made. Another diagnosis considered based upon the location of the tumor was greater superficial petrosal nerve (GSPN) schwannoma. The patient was taken up for right temporal craniotomy and tumor excision upon pre-anesthetic clearance.
With the patient in the lateral position, a right temporal craniotomy extending to the base of the middle cranial fossa was made. No mastoid air cells were encountered during the bone work. The tumor was excised by extradural approach and was found to be grey-colored, well defined and encapsulated, and of variable consistency with areas of necrosis. Anterior petrosal bone was found to be eroded at the area of tumoral attachment. No drilling of the petrosal bone was done during the procedure. Nerve monitoring was not employed. Intraoperative frozen section was reported as round to spindle cell tumor after the surgery; the patient was extubated and shifted to intensive care unit for further management.
The patient complained of decreased hearing in the right ear on the first postoperative day. The patient was mobilized the same day and had no significant complaints otherwise. Barring CN VIII, the rest of the CN examination was normal. Aminoglycoside antibiotics and nonsteroidal anti-inflammatory drug were with-held as these drugs are ototoxic. On the third postoperative day, the patient was discharged at his insistence. He was prescribed tapering doses of steroids and advised to follow up in one week.
The patient was reviewed the following week after discharge. Other than the only complaint of hearing loss, he was recovering well, with no significant headache or seizure episode. The histopathology report was obtained, findings of which were reflective of schwannoma with no features of malignancy, contrary to the provisional diagnosis of meningioma. Histopathology examination revealed 1) spindle cells arranged in fascicles with some cells showing degenerative atypia and 2) round to oval cells with clear cytoplasm. Coagulative necrosis with macrophages infiltration was present. Mitosis was rare. On immunohistochemistry analysis, tumor tissue was positive for vimentin and S-100. While epithelial membrane antigen, progesterone receptor, smooth muscle actin, and glial fibrillary acidic protein were found to be negative. The Ki-labelling index was seen to be 6%–7% (Fig. 2).
Both histopathology findings and hearing loss were unexpected outcomes in this patient. PTA and BERA were done in the following weeks (Supplementary Fig. 1 in the online-only Data Supplement). PTA was suggestive of moderate sensory neural hearing loss with a slightly mixed component at lower frequencies in the right ear. BERA was not recordable on the right side while normal on the left side, suggesting retrochoclear hearing loss. The patient is now on follow-up with otorhinolaryngologist for hearing rehabilitation.
Acoustic neuromas are the most common lesion at the cerebellopontine angle [4]. Acoustic schwannoma most commonly presents with symptoms of auditory disturbance (hearing loss, tinnitus), disequilibrium and vertigo along with headache. Cerebellar and cranial nerve (CN V, VII and lower cranial nerves) dysfunction is also known to occur [1]. Symptoms of CN V, VI, VII, IX, X, XI, and XII involvement can also occur depending on tumor extent.
Intra cerebral schwannomas account for less than 1% of intracranial schwannoma [5]. In a paper published by Gao et al. [6] in 2017, 84 cases of intracerebral schwannoma were reviewed, of which 61 cases were supratentorial in location. Such schwannomas are not associated with any CN [7].
Intraosseous schwannoma account for less than 0.2% of primary bone tumors is another unusual presentation of schwannoma [8]. Only 10 such cases of intraosseous schwannoma have been reported [9]. Schwannomas arising from the posterior fossa, jugular foramen, or Meckel’s cave are known to involve the petrous apex [10]. Temporal bone erosion by acoustic schwannomas is a rare feature, mentioned only in a couple of case reports [1112].
While reviewing the literature, the authors came across a case report of a patient diagnosed with meningioma of the right middle cranial fossa arising from the petrous bone. After the surgery patient developed facial palsy. Histopathology revealed the tumor to be a malignant nerve sheath tumor; in contrast, the biopsy, in our case, revealed schwannoma, and the patient developed hearing loss [13].
The malignant peripheral nerve sheath tumors (MPNST) are uncommon entities (0.001%) that occur even more rarely intracranially [14].
Intracranial extra-axial involve CNs, most commonly CN VIII. MPNST can occur de novo or may occur as a malignant transformation of the pre-existing benign tumors like schwannoma [15].
The patient presenting as a skull base meningioma was ultimately diagnosed with CN VIII schwannoma, a highly unusual occurrence. Histopathology and IHC were conclusive of benign schwannoma. Explanation of hearing loss proved to be a challenge for the operating team. It did hamper the quality of life of the patient, a young professional.
To evaluate the immediate postoperative hearing loss, BERA and PTA were done to localize the cause of hearing loss. BERA was diagnostic of retrocochlear hearing loss.
In specific neurosurgical procedures like microvascular decompression, conductive hearing loss has been reported owing to fluid entering the mastoid air cells during craniotomy [16].
In cases of conductive hearing loss due to middle ear and mastoid effusion, the timing of the audiogram is crucial as it takes about three months for the effusion to resolve [17].
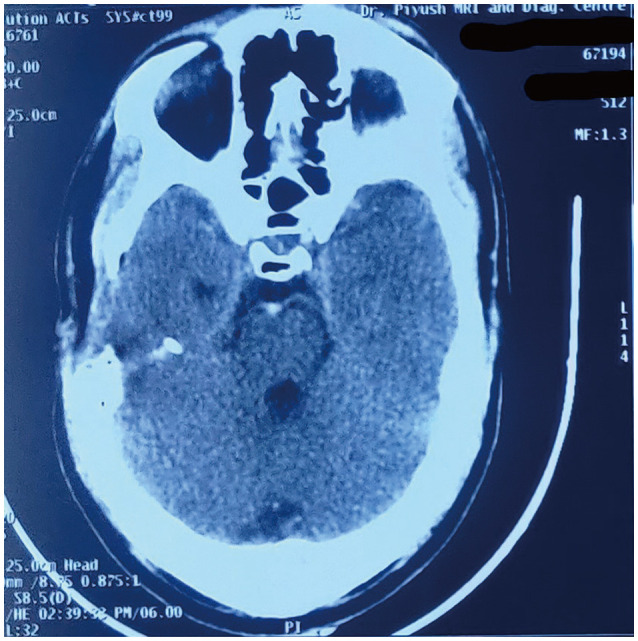
Postoperative noncontrast CT head showed erosion of the anterior petrous bone, also observed intraoperatively (Fig. 3).
The possibility of infiltration of the mass lesion into the inner ear is another possibility to be considered for hearing loss, but there was no evidence of hearing loss in the preoperative examination. Intraoperative damage to the inner ear is unlikely as the petrous bone was not drilled.
Given the location of the tumor, GSPN schwannoma is a possibility to consider. Uppar et al. [18] reviewed 27 cases of GSPN schwannoma. These cases presented most commonly with a history of facial palsy (16 cases) or hearing loss (14 cases); other less commonly occurring symptoms included seizures, headache, ocular pain, and irritation. In all these cases, the tumor was located in the middle cranial fossa and subtemporal approach with an extradural or intradural approach to follow. No new-onset postoperative hearing loss was not found in any of these patients, unlike in our case, in whom the loss of hearing without facial palsy and xerophthalmia occurred.
Intracranial malignant nerve sheath tumor has been reported to be associated with cranial nerves; our observation of an atypical schwannoma involving the eighth cranial nerve is first of a kind. As per the authors’ best knowledge, such is the first case report of acoustic schwannoma with such an atypical presentation.
In such a scenario, preoperative history, clinical examination, and imaging have failed to diagnose, leading to an unexpected complication.
Intracerebral schwannomas unrelated to CN have been reported. Our case is that of an atypical schwannoma masqueraded as meningioma of the skull base. The immediate retrocochlear sensorineural hearing loss points to the origin from the CN VIII. Such a case is one of a kind, proving to be a diagnostic challenge.
Notes
Author Contributions:
Conceptualization: Sanjeev Dua.
Data curation: Vikrant Katyar.
Formal analysis: Hershdeep Singh.
Investigation: Hershdeep Singh.
Methodology: Anil Dhar.
Project administration: Sanjeev Dua.
Resources: Hershdeep Singh.
Supervision: Anil Dhar.
Visualization: Hershdeep Singh.
Writing—original draft: Hershdeep Singh.
Writing—review & editing: all authors.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article.
References
1. Sanna M, Mancini F, Russo A, Taibah A, Falcioni M, Di Trapani G. Atlas of acoustic neurinoma microsurgery. 2nd ed. Stuttgart: Thieme;2011.
2. Silk PS, Lane JI, Driscoll CL. Surgical approaches to vestibular schwannomas: what the radiologist needs to know. Radiographics. 2009; 29:1955–1970. PMID: 19926756.

3. Kumar V, Abbas AK, Aster JC. Robbins and Cotran pathologic basis of disease. 9th ed. Philadelphia: Elsevier Saunders;2015.
4. Lanser MJ, Sussman SA, Frazer K. Epidemiology, pathogenesis, and genetics of acoustic tumors. Otolaryngol Clin North Am. 1992; 25:499–520. PMID: 1625863.

5. Russels DS, Rubinstein LJ. Pathology of tumors of the nervous system. 5th ed. London: Edward Arnold;1989.
6. Gao Y, Qin Z, Li D, Yu W, Sun L, Liu N, et al. Intracerebral schwannoma: a case report and literature review. Oncol Lett. 2018; 16:2501–2510. PMID: 30013644.

7. Rotondo M, Pascale M, Scuotto A. Giant frontal intraparenchymal schwannoma: unexpected diagnosis. BMJ Case Rep. 2013; 2013:bcr2013010350.
8. Bansal AK, Bindal R, Shetty DC, Dua M. Rare occurrence of intraosseous schwannoma in a young child, its review and its pathogenesis. J Oral Maxillofac Pathol. 2012; 16:91–96. PMID: 22438647.

9. Rais F, Benhmidou N, Rais G, Kouhen F, Bellahamou K, Loughlimi H, et al. Solitary intraosseous schwannoma of the base and vault of the skull: a summary review of such unusual location. Clin Sarcoma Res. 2015; 5:6. PMID: 25667745.
10. Jackler RK, Parker DA. Radiographic differential diagnosis of petrous apex lesions. Am J Otol. 1992; 13:561–574. PMID: 1449185.
11. Feghali JG, Kantrowitz AB. Atypical invasion of the temporal bone in vestibular schwannoma. Skull Base Surg. 1995; 5:33–36. PMID: 17171155.

12. Park SJ, Yang NR, Seo EK. Vestibular schwannoma atypically invading temporal bone. J Korean Neurosurg Soc. 2015; 57:292–294. PMID: 25932298.
13. Patankar AP, Sheth JH. Intracranial malignant nerve sheath tumor in the middle cranial fossa: a rare case report with review of literature. Asian J Neurosurg. 2019; 14:922–926. PMID: 31497130.
14. Ziadi A, Saliba I. Malignant peripheral nerve sheath tumor of intracranial nerve: a case series review. Auris Nasus Larynx. 2010; 37:539–545. PMID: 20399579.
15. L’heureux-Lebeau B, Saliba I. Updates on the diagnosis and treatment of intracranial nerve malignant peripheral nerve sheath tumors. Onco Targets Ther. 2013; 6:459–470. PMID: 23667313.
16. Watanabe T, Saito N, Sato N, Takahashi A, Fujimaki H, Tosaka M, et al. High incidence and spontaneous resolution of mastoid effusion after craniotomy on early postoperative magnetic resonance images. Neuroradiology. 2003; 45:482–488. PMID: 12811440.
17. Ying T, Thirumala P, Gardner P, Habeych M, Crammond D, Balzer J. The incidence of early postoperative conductive hearing loss after microvascular decompression of hemifacial spasm. J Neurol Surg B Skull Base. 2015; 76:411–415. PMID: 26682118.
18. Uppar AM, Rao S, Prasad C, Arimappamagan A, Santosh V. Schwannoma of the greater superficial petrosal nerve: an unusual site for a common tumor. J Neurol Surg A Cent Eur Neurosurg. 2020; 81:565–570. PMID: 32361981.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.14791/btrt.2022.10.e21.
Supplementary Fig. 1
Pure tone audiometry suggestive of right ear moderate senosorineural hearing loss with slight mixed component at lower frequencies. Brainstem evoked response audiometry suggestive of retrochochlear sensorineural hearing loss.
Fig. 1
Contrast-enhanced MRI sagittal, axial, and coronal sections showing tumor arising from right middle cranial fossa.
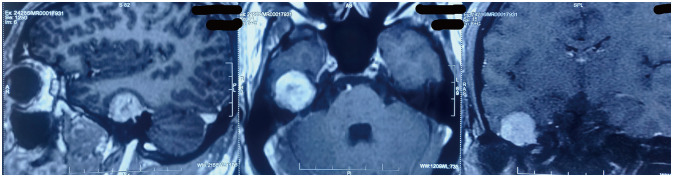
Fig. 2
Histopathological finding. A: H&E section (×10) shows a biphasic tumor comprised of oval to spindle shaped cells with cellular component (Antoni A area) and myxoid hypocellular component (Antoni B area). B: H&E section (×40) shows tumor comprised of cells are narrow, elongated and wavy with tapered ends interspersed with collagen fibers with an occasional mitotic figure. C: Section examined shows diffuse strong (4+) cytoplasmic positivity of tumor cells for vimentin on immunohistochemistry (×40). D: Section examined shows diffuse cytoplasmic and focal nuclear positivity of tumor cells for S-100 on immunohistochemistry (×10).





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download