This article has been
cited by other articles in ScienceCentral.
Abstract
Spontaneous regression of meningioma is rarely observed. We report a one person of an incidentally diagnosed meningioma with a spontaneous regression. The 73-year-old female patient without symptoms showed the right sphenoid meningioma with peritumoral edema. The meningioma was incidentally diagnosed and followed up by MRI for 10 years. The tumor shrank with a decrease of edema on T2 MRI. The initial volume of 58.59 cm3, regressed to 37.16 cm3.
Keywords: Meningioma, Spontaneous regression, Peritumoral edema, diabetes mellitus
INTRODUCTION
Spontaneous regression of tumors is defined as spontaneous remission of a tumor in the absence of any treatments [
1]. Although the mechanisms of spontaneous regression remain unclear, they are known to include infection, apoptosis, or immune system activation [
23]. Meningiomas, originating from the arachnoid cap cell, are the most common primary intracranial tumors in adults [
45]. Meningiomas are generally slow-growing tumors [
5]. But spontaneous regression of meningiomas has been rarely reported. We report a case of an incidentally diagnosed meningioma with spontaneous regression after 10 years.
CASE REPORT
A 73-year-old female patient with an incidentally diagnosed meningioma was introduced to our hospital in 2012. She was diagnosed with type 2 diabetes mellitus (DM). She had no other past history of infections. There were no neurologic symptoms nor subjective complaints related to the tumor. Contrast-enhanced brain MRI revealed a large meningioma (47.04×40.18×62.31 mm) of the right sphenoid (
Fig. 1A). This meningioma showed a high intensity on T2 MRI with significant edema (
Fig. 2A). Blood sugar had been well controlled with medication-metformin. We planned operation. However, the patient refused surgical treatment. She was lost to follow-up at the outpatient department.
Ten years after, she was admitted to our hospital after checked contrast-enhanced brain MRI in 2022 due to decreased cognitive function five years ago. Blood sugar had been well controlled with medication-metformin. The MRI showed shrinkage of the meningioma to a size of 42.66×31.72×54.92 mm (
Fig. 1B). This meningioma showed decreased edema on T2 MRI (
Fig. 2B). She had not received any treatment such as gamma knife surgery, for meningioma. The initial volume (=length×depth×width×0.5) [
6] was 58.59 cm
3. It regressed to 37.16 cm
3 10 years later. Since there were no other neurological symptoms, we decided to follow up this patient later.
DISCUSSION
Meningiomas are the most frequently diagnosed primary brain tumors [
7]. Meningioma tumor growth shows a curve pattern [
8]. But there have been very few reports of meningioma regressing spontaneously.
Kumaria et al. [
9] have reported one patient with spontaneous regression of meningioma. They reported that the size of the meningioma was reduced from 36.0 cm
3 to 11.2 cm
3 over 7 years. In their study, although the mechanism was unclear, they considered that menopause might have induced tumor shrinkage. They also mentioned that microangiopathic changes on a histological level might have contributed to tumor shrinkage.
Hirota et al. [
10] have reported one case of spontaneous regression of meningioma. They reported that the size of meningioma was reduced from 25.5 cm
3 to 9.9 cm
3 over 7 years. In their study, they considered that uncontrolled DM and insulin might be probable causes of the regression spontaneously.
Our hypothesis is that calcification of arteries can occur due to old age and DM, followed by a decrease in blood supply in tumor, which leads to a decrease in tumor size. DM causes various complications, and the association between DM and brain damage has been widely reported. According to Shukla et al. [
11], DM increases the risk of cerebral ischemia. They reported that DM induced atherosclerosis, which might have increased the risk of ischemic stroke. DM was also present in our case, and it was presumed that the mass size was reduced by DM.
Atherosclerosis seen in spontaneous tumor regression might be similar to the effect after embolization in meningiomas. Several studies have reported a reduction in tumor volume following embolization, showing substantial tumor shrinkage after embolization feeders [
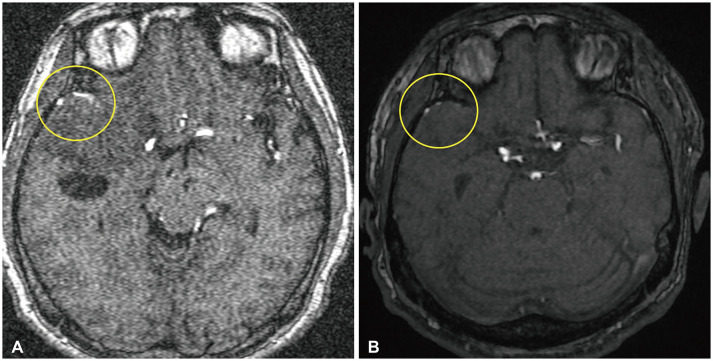
12131415]. Although there is no clear blood vessel study such as cerebral angiography, our case showed that arteries on magnetic resonance angiography were less visible after tumor regression (
Fig. 3).
In our case, a decrease in peritumoral edema was also seen as the tumor size decreased. We hypothesize that the decrease in peritumoral edema is the result of activation of collateral vessels. The relationship between peritumoral edema and collateral vessels has been described in other paper [
16]. The function of collateral vessels pressed by tumor might be normalized due to the regression of tumor size, leading to a decrease in peritumoral edema. We also confirmed the regression of a peritumoral cyst. Although the exact cause of peritumoral cyst in meningioma has not been elucidated, an increase in collateral vessel function might be associated with a decrease in cyst.
Our study had several limitations. First, it is difficult to generalize our hypothesis as we report only one case. Second, no pathological tests were made to support the hypothesis. Further research will be needed on this point.
We reported a case of spontaneous regression meningioma. Through this case, if the patient is asymptomatic, further discussions should be considered whether early operation should be performed for large meningioma. And further studies are needed on the relationship between DM and spontaneous regression of meningioma.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Cole WH, Everson TC. Spontaneous regression of cancer: preliminary report. Ann Surg. 1956; 144:366–383. PMID:
13363274.
2. Salman T. Spontaneous tumor regression. J Oncol Sci. 2016; 2:1–4.

3. Hoption Cann SA, van Netten JP, van Netten C, Glover DW. Spontaneous regression: a hidden treasure buried in time. Med Hypotheses. 2002; 58:115–119. PMID:
11812185.
4. Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R, et al. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021; 597:119–125. PMID:
34433969.

5. Fountain DM, Soon WC, Matys T, Guilfoyle MR, Kirollos R, Santarius T. Volumetric growth rates of meningioma and its correlation with histological diagnosis and clinical outcome: a systematic review. Acta Neurochir (Wien). 2017; 159:435–445. PMID:
28101641.

6. Kasuya H, Kubo O, Kato K, Krischek B. Histological characteristics of incidentally-found growing meningiomas. J Med Invest. 2012; 59:241–245. PMID:
23037194.

7. Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005; 57:1088–1095. PMID:
16331155.

8. Nakasu S, Nakasu Y. Natural history of meningiomas: review with meta-analyses. Neurol Med Chir (Tokyo). 2020; 60:109–120. PMID:
32009127.

9. Kumaria A, Ingale HA, Macarthur DC. Spontaneous regression of a large skull base meningioma: case report. Br J Neurosurg. 2020; 34:205–206. PMID:
29334772.
10. Hirota K, Fujita T, Akagawa H, Onda H, Kasuya H. Spontaneous regression together with increased calcification of incidental meningioma. Surg Neurol Int. 2014; 5:73. PMID:
24949216.
11. Shukla V, Shakya AK, Perez-Pinzon MA, Dave KR. Cerebral ischemic damage in diabetes: an inflammatory perspective. J Neuroinflammation. 2017; 14:21. PMID:
28115020.

12. Bendszus M, Martin-Schrader I, Schlake HP, Solymosi L. Embolisation of intracranial meningiomas without subsequent surgery. Neuroradiology. 2003; 45:451–455. PMID:
12802546.
13. Bendszus M, Martin-Schrader I, Warmuth-Metz M, Hofmann E, Solymosi L. MR imaging- and MR spectroscopy-revealed changes in meningiomas for which embolization was performed without subsequent surgery. AJNR Am J Neuroradiol. 2000; 21:666–669. PMID:
10782775.
14. Koike T, Sasaki O, Tanaka R, Arai H. Long-term results in a case of meningioma treated by embolization alone--case report. Neurol Med Chir (Tokyo). 1990; 30:173–177. PMID:
1697044.
15. Wakhloo AK, Juengling FD, Van Velthoven V, Schumacher M, Hennig J, Schwechheimer K. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol. 1993; 14:571–582. PMID:
8517342.
16. Kim JH, Moon KS, Jung JH, Jang WY, Jung TY, Kim IY, et al. Importance of collateral venous circulation on indocyanine green videoangiography in intracranial meningioma resection: direct evidence for venous compression theory in peritumoral edema formation. J Neurosurg. 2019; 132:1715–1723. PMID:
31125964.
Fig. 1
Contrast-enhanced brain MRI axial views. A: In 2012, the size of meningioma was 47.04×40.18×62.31 mm. B: 10 years later in 2022, the size of meningioma was 42.66×31.72×54.92 mm.
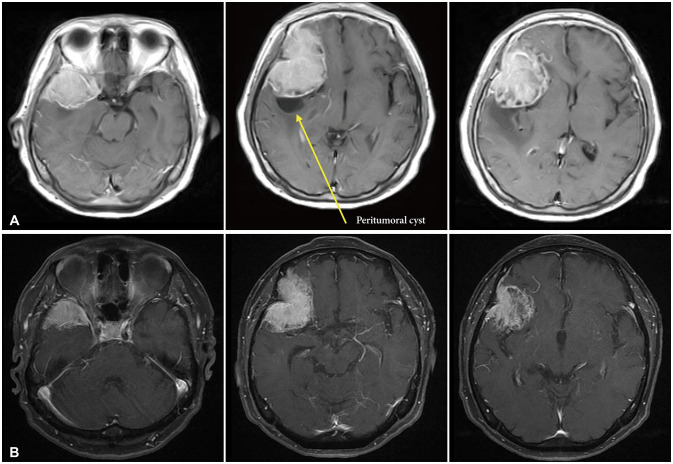
Fig. 2
T2 MRI axial views. A: MRI taken in 2012. The peritumoral edema was seen at temporal area. B: MRI taken in 2022. The peritumoral edema was almost invisible.
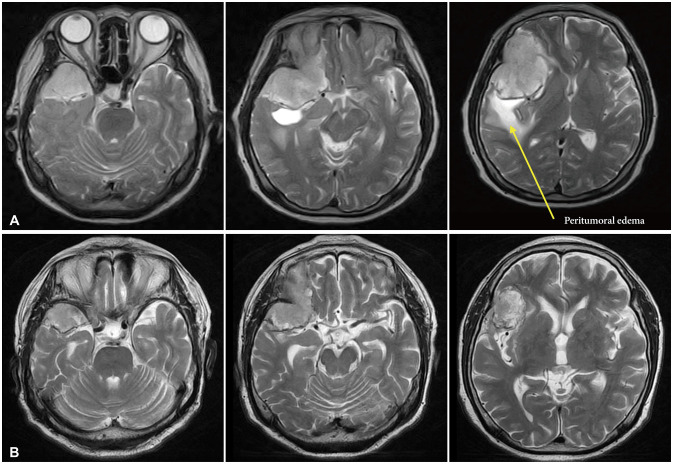
Fig. 3
Comparison of MR angiography views before and after tumor shrinkage. Yellow circle indicated the feeding arteries. A: MR angiography taken in 2012. The feeding artery was observed well. B: MR angiography taken in 2022. The feeding artery was thinly observed.





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download