Abstract
Background
Extraventricular neurocytoma (EVN) is an extremely rare neuronal neoplasm that arises outside the ventricle. The clinical implication of the heterogenous prognosis of this rare tumor has not yet been clarified. Herein, we analyzed our institutional series of EVN.
Methods
A total of eight consecutive cases were enrolled and investigated. The prognosis of EVN was analyzed and compared to that of central neurocytoma (CN).
Results
There were two male and six female patients, and the median age was 36.5 years. The median tumor size was 38 mm, and the most common location of the tumor was the frontal lobe (3, 37.5%), followed by the parietal and temporal lobes. In brain imaging, four (50%) tumors showed peritumoral edema and three (37.5%) tumors showed calcification. All patients underwent gross total resection, and two (25%) underwent adjuvant radiotherapy. The 5-year overall survival (OS) was 55.6%, and the 2-year progression-free survival (PFS) was 42.9%. The OS and PFS of EVN were poor compared to those of CN. Although EVN is a single disease entity, individual patients showed varying prognosis. One patient showed no recurrence during the 7-year follow-up period; however, another patient had a recurrence 4 months after surgery and died 2 years later.
Extraventricular neurocytomas (EVNs) are rare neuronal neoplasms, designated as a World Health Organization (WHO) grade 2 tumor in 2007. Central neurocytoma (CN) was first described by Hassoun et al. [1] in 1982. EVN, which is histologically identical to CN, occurs in the brain parenchyma. Radiologically, EVNs are commonly misdiagnosed as oligodendroglioma or ependymoma [23]. Atypical EVN can be diagnosed when the tumor shows histological atypia or a Ki-67 labeling index of over 2% [4]. Owing to its rarity, the treatment consensus, precise prognosis, and treatment outcome are not well known. Thus, we reviewed our institutional series of EVN.
This study was approved by the Institutional Review Board of Asan Asan Medical Center (approval no. 2021-1137). Informed consent was waived due to the retrospective nature of the study. We searched our institutional database for EVN diagnoses made between January 2005 and December 2020. Only newly diagnosed patients and those who underwent surgery at our hospital were enrolled in the current study; patients with recurrent tumors and no available information on the primary tumor were excluded.
A total of 10 EVN patients were initially included in the study population, but 2 of them were later excluded due to a lack of data. In terms of histopathological examination, microscopically, monotonous bland cells with modest cytoplasm and “salt and pepper”-like chromatin is characteristics of EVN. Astrocytic tumor could be misdiagnosed as EVN. Histopathological re-evaluation was performed to exclude misdiagnosis for all patient. If there was histological atypia or the MIB-1 labeling index was greater than 2%, our institutional neuropathologist re-classified the lesions as typical or atypical EVN [5]. The size of the tumor was defined by its maximal diameter in two dimensions.
The extent of resection was defined as gross total resection (GTR) if total removal was confirmed by postoperative MRI, or as subtotal resection (STR) for any degree of incomplete resection of the tumor. Adjuvant postoperative typical radiation therapy (RT) was administered for residual tumors at a dose range of 54–56 Gy, fractionated by 2 Gy. Initial follow-ups involved clinical evaluations and an MRI in the immediate postoperative period (within 72 hours), as well as 1 month, 6 months, and 1 year after surgery, with annual examinations thereafter.
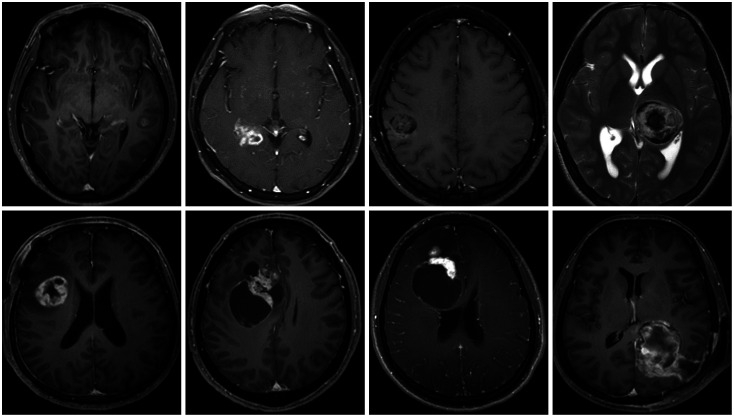
This study included two male and six female patients. The median age was 36.5 years (range: 14–72 years), and the median tumor size was 38 mm. The most common location of the tumor was the frontal lobe, followed by the parietal lobe, temporal lobe, and thalamus. Half of the tumors showed peritumoral edema in brain imaging, and three of eight patients had intratumoral calcification. The median follow-up period was 38 months. Detailed characteristics of the enrolled patients are presented in Tables 1 and 2. Initial imaging findings of the enrolled patients are presented in Fig. 1.
All patients underwent GTR of tumors, and there were no postoperative complications. Hemiparesis occurred during the resection of thalamic tumors. Atypical histology was noted in four of eight (50%) patients. Adjuvant RT was performed in two of four atypical tumors. During the follow-up period, four of eight (50%) patients had tumor recurrence and two of eight (25%) patients died (Table 3). In terms of recurrence, all of first recurrence was local recurrence. Also, all recurrent tumors underwent redo-resection. In histological examination, all recurrent tumors were diagnosed as neurocytoma. Two died patients showed multiple distant recurrence and leptomeningeal seeding after second surgery. In these two patients, malignant transformation to higher grade glioma was suspicious, however, biopsy was not undergone. The treatment outcome varied. Typical EVN showed a favorable long-term outcome (Fig. 2), whereas atypical EVN showed a poor outcome, and we encountered one case with very aggressive features (Fig. 3). The 2-year OS was 100%, and the 3-year OS was 83.3%. The 6-month PFS was 85.7%, and the 2-year PFS was 42.9% (Fig. 4).
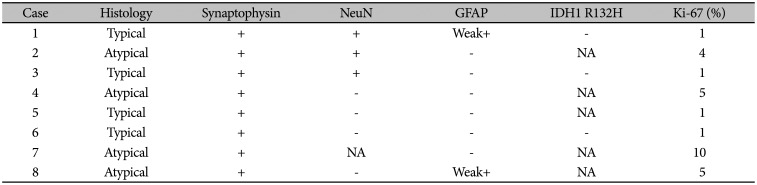
EVN showed monotonous bland cells with modest cytoplasm and “salt and pepper”-like chromatin. They showed no signs of necrosis or endothelial proliferation (Fig. 5). All tumors showed ‘synaptophysin’ positivity, and three of seven tumors showed ‘NeuN’ positivity. Only two of eight tumors showed weak positivity for glial fibrillary acidic protein. All atypical EVNs showed a Ki-67 labeling index of over 2%. The immunohistochemical findings are presented in Table 4.
EVN is a very rare central nervous system tumor that occurs in young adults. EVN has been detected in various sites outside the ventricular system, including the cerebral hemisphere and deep nuclei. It can also involve the cerebellum, brainstem, and spinal cord [67]. The 2007 WHO classification included EVN as a brain tumor entity to distinguish it from CN [8]. However, EVN has not been revised or re-classified in the 2016 and 2021 WHO classification [9].
On MRI, most tumors were solid-cystic or solid and were sometimes accompanied by multiple small cysts called “soap-bubbles” [3]. The tumors were often well circumscribed and contrast enhanced [3]. However, there was no pathognomonic radiological finding; EVN usually mimics other astrocytic glial tumors. Cyst degeneration, calcification, and perilesional edema were observed in EVN [7]. On advanced MRI, spectroscopy showed a prominent choline peak and decreased N-acetylaspartate; perfusion is usually increased in solid tumors [210].
EVN exhibited a wide spectrum of morphologies, cellularity, and proliferation rates despite a strong tendency toward ganglionic or glial differentiation [6]. Synaptophysin is considered the most specific marker for EVN [11]. Multiple perivascular pseudorosettes originate from tumor cells arranged irregularly around blood vessels with or without perivascular anucleate zones, forming synaptophysin in EVN [412]. In our series, all patients showed positivity for synaptophysin. Xu et al. [6] reported that MAP-2 may also be a specific marker for EVN. Furthermore, while EVN does not usually express IDH R132H, astrocytic and oligodendroglial tumors do, and this can be an important differential diagnostic feature [13]. In 2021 WHO classification, genetic alteration in FGFR1-TACC1 fusion and IDH R132H wildtype are key findings to establish EVN diagnosis [13]. In South Korea, evaluating next generation sequencing (NGS) for brain tumor was approved to cover Korean National Health Insurance after 2017. Unfortunately, all patients in our study were diagnosed with EVN before 2017. Thus, our study lacks next generation sequencing data.
Atypical features of EVN include nuclear atypia, increased mitotic figures, focal necrosis, and high Ki-67 labeling index [61214]. Atypical EVN has not been assigned a WHO grade. In CN, the atypical form shows a high recurrence rate, but it does not affect survival [15]. Atypical EVN could behave aggressively and have an overall poor prognosis with a higher recurrence rate [2]. Kane et al. [4] reported that atypical histology showed a 3-fold higher recurrence rate and 10-fold higher chance of mortality. This result is consistent with our series. In our study, one case showed rapid tumor recurrence and dismal prognosis, and all patients who died had atypical EVN.
The treatment consensus of EVN has not yet been established, and treatment of atypical EVN has not been distinguished from treatment of typical EVN. Complete resection is the ideal treatment for neurocytoma [151617]. In CN, STR with adjuvant RT showed comparable treatment outcomes with GTR [15]. However, the outcomes of EVN tumor have not yet been clarified. Moreover, the treatment of atypical EVN with a poor prognosis has not been differentiated from that of typical EVN.
Gamma knife radiosurgery (GKS) showed a favorable outcome in CN as a primary or adjuvant treatment. However, there has been a lack of data for EVN owing to its extremely low incidence [1518]. Byun et al. [15] reported a favorable treatment outcome of GKS for residual tumor after CN surgery. In their report, CyberKnife radiosurgery was used as an adjuvant therapy for subtotal-resected CN. Jeon et al. [18] reported a favorable outcome of GKS for radiologically diagnosed CN and suggested that GKS can be used as a primary treatment for small CNs.
There has been a lack of evidence for chemotherapy for both typical and atypical EVN. Brandes et al. [19] reported a favorable outcome of chemotherapy in progressive and recurrent CN; they used a platinum-based agent in three patients. However, there is a lack of data on chemotherapy for both typical and atypical EVN. In our series, one patient with atypical EVN received platinum-based chemotherapy for progressive recurrent disease; however, the tumor progressed continuously during chemotherapy.
This study has a few limitations. First, it was a retrospective study that included only eight patients with EVN, which, combined with the limitations inherent in any retrospective design, precluded any meaningful multivariate analysis of survival outcomes or risk factors. Second, since this study series spanned over 15 years, the treatment methods varied. NGS for brain tumor now is essential for establishing accurate diagnosis. Although morphological diagnosis is established, it may be changed following the result of NGS genetic alterations. However, NGS could not be performed in our study. In addition, no alternative treatment strategy has been developed for atypical EVN, making it difficult to draw any conclusions regarding the optimal intervention strategies and outcomes.
EVNs are extremely rare neoplasms that differ from CNs. The prognosis of EVN is variable, and atypical histology may be an important prognostic factor. Therefore, we believe that the data from our current single-center series would significantly contribute to the existing literature on these extremely rare tumors, as well as to future meta-analyses of this disease.
Acknowledgments
We thank the referring physicians, our institutional neuroradiologists and pathologists. We also thank Minji Rickie Kim (neurologist) and Woongjae Jeon (nephrologist) who gave us warm advice and help.
Notes
Author Contributions:
Conceptualization: Joonho Byun.
Data curation: Joonho Byun.
Formal analysis: Joonho Byun.
Investigation: Joonho Byun, Moinay Kim.
Methodology: Joonho Byun.
Project administration: Joonho Byun.
Supervision: Sang Woo Song, Young-Hoon Kim, Chang Ki Hong, Jeong Hoon Kim.
Validation: Sang Woo Song, Young-Hoon Kim, Chang Ki Hong, Jeong Hoon Kim.
Visualization: Joonho Byun.
Writing—original draft: Joonho Byun.
Writing—review & editing: all authors.
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available due to privacy and ethical concerns but are available from the corresponding author on reasonable request.
References
1. Hassoun J, Gambarelli D, Grisoli F, Pellet W, Salamon G, Pellissier JF, et al. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982; 56:151–156. PMID: 7064664.
2. Patil AS, Menon G, Easwer HV, Nair S. Extraventricular neurocytoma, a comprehensive review. Acta Neurochir (Wien). 2014; 156:349–354. PMID: 24357019.

3. Yang GF, Wu SY, Zhang LJ, Lu GM, Tian W, Shah K. Imaging findings of extraventricular neurocytoma: report of 3 cases and review of the literature. AJNR Am J Neuroradiol. 2009; 30:581–585. PMID: 18842767.

4. Kane AJ, Sughrue ME, Rutkowski MJ, Aranda D, Mills SA, Lehil M, et al. Atypia predicting prognosis for intracranial extraventricular neurocytomas. J Neurosurg. 2012; 116:349–354. PMID: 22054208.

5. Söylemezoglu F, Scheithauer BW, Esteve J, Kleihues P. Atypical central neurocytoma. J Neuropathol Exp Neurol. 1997; 56:551–556. PMID: 9143268.

6. Xu L, Ouyang Z, Wang J, Liu Z, Fang J, Du J, et al. A clinicopathologic study of extraventricular neurocytoma. J Neurooncol. 2017; 132:75–82. PMID: 27864704.

7. Liu K, Wen G, Lv XF, Deng YJ, Deng YJ, Hou GQ, et al. MR imaging of cerebral extraventricular neurocytoma: a report of 9 cases. AJNR Am J Neuroradiol. 2013; 34:541–546. PMID: 23042917.

8. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109. PMID: 17618441.

9. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820. PMID: 27157931.

10. Möller-Hartmann W, Krings T, Brunn A, Korinth M, Thron A. Proton magnetic resonance spectroscopy of neurocytoma outside the ventricular region--case report and review of the literature. Neuroradiology. 2014; 44:230–234.

11. Brown DM, Karlovits S, Lee LH, Kim K, Rothfus WE, Brown HG. Management of neurocytomas: case report and review of the literature. Am J Clin Oncol. 2001; 24:272–278. PMID: 11404499.
12. Choi H, Park SH, Kim DG, Paek SH. Atypical extraventricular neurocytoma. J Korean Neurosurg Soc. 2011; 50:381–384. PMID: 22200023.

13. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23:1231–1251. PMID: 34185076.

14. Brat DJ, Scheithauer BW, Eberhart CG, Burger PC. Extraventricular neurocytomas: pathologic features and clinical outcome. Am J Surg Pathol. 2001; 25:1252–1260. PMID: 11688459.
15. Byun J, Hong SH, Yoon MJ, Kwon SM, Cho YH, Kim JH, et al. Prognosis and treatment outcomes of central neurocytomas: clinical interrogation based on a single center experience. J Neurooncol. 2018; 140:669–677. PMID: 30225773.

16. Rades D, Schild SE, Fehlauer F. Defining the best available treatment for neurocytomas in children. Cancer. 2004; 101:2629–2632. PMID: 15494975.

17. Rades D, Fehlauer F, Schild SE. Treatment of atypical neurocytomas. Cancer. 2004; 100:814–817. PMID: 14770439.

18. Jeon C, Cho KR, Choi JW, Kong DS, Seol HJ, Nam DH, et al. Gamma Knife radiosurgery as a primary treatment for central neurocytoma. J Neurosurg. 2021; 134:1459–1465.

19. Brandes AA, Amistà P, Gardiman M, Volpin L, Danieli D, Guglielmi B, et al. Chemotherapy in patients with recurrent and progressive central neurocytoma. Cancer. 2000; 88:169–174. PMID: 10618620.

Fig. 2
Representative cases of treatment outcome of typical extraventricular neurocytoma. A and B: Brain MRI of a 20-year-old female patient (case 5) showing a large solid cystic mass in the right frontal lobe. The mass shows heterogenous enhancement (A). Total tumor resection is performed, and there is no recurrence during the 7-year follow-up period (B). C and D: Brain MRI of a 58-year-old male patient (case 2) showing a large solid cystic mass in the right frontal lobe (C). The tumor is totally resected, and there is no recurrence during the 6-year follow-up period (D).
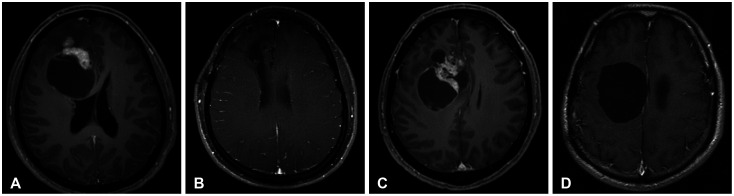
Fig. 3
Image findings of the worst prognosis of atypical extraventricular neurocytoma. A 41-year-old female patient (case 8) with left parieto-occipital tumor undergoing tumor resection through a transcortical approach. A: Initial brain MRI. B: Transcortical approach is performed. Histopathological examination reveals that it is an atypical EVN. C: On the 4-month follow-up MRI, tumor recurrence is noted. D: Revisional craniotomy and redo-resection of the tumor is performed. Subsequently, adjuvant radiation therapy is administered. E and F: However, rapid recurrence is observed in the 3-month follow-up MRI. Ifosfamide, carboplatin, and etoposide systemic chemotherapy (ICE chemo) regimen are prescribed. G: However, continuous tumor progression is noted despite the chemotherapy. EVN, extraventricular neurocytoma; GTR, gross total resection; RT, radiation therapy.
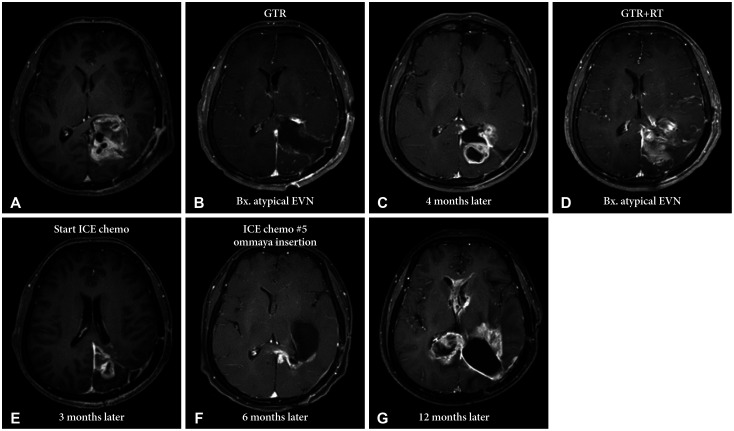
Fig. 4
Kaplan-Meier survival analysis of extraventricular neurocytoma. Two-year overall survival (OS) of the enrolled patients with extraventricular neurocytoma is 100%, and the 5-year OS is 55.6%. The 6-month progression-free survival (PFS) of the enrolled patients with extraventricular neurocytoma is 85.7%, and the 2-year PFS is 42.9%.
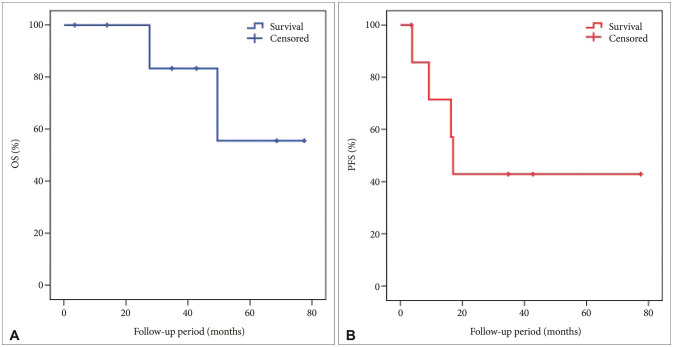
Fig. 5
Histopathological findings of extraventricular neurocytoma (EVN). A and B: Tumor cells showing sheets, clusters, and rosettes of monotonous cells with round nuclei and distinct nucleoli (A: hematoxylin-eosin [HE] stain, ×40, B: HE stain, ×100). C: EVN exhibiting uniform staining for synaptophysin. D: The Ki-67 index is 5%; thus, the patient is diagnosed with atypical EVN.
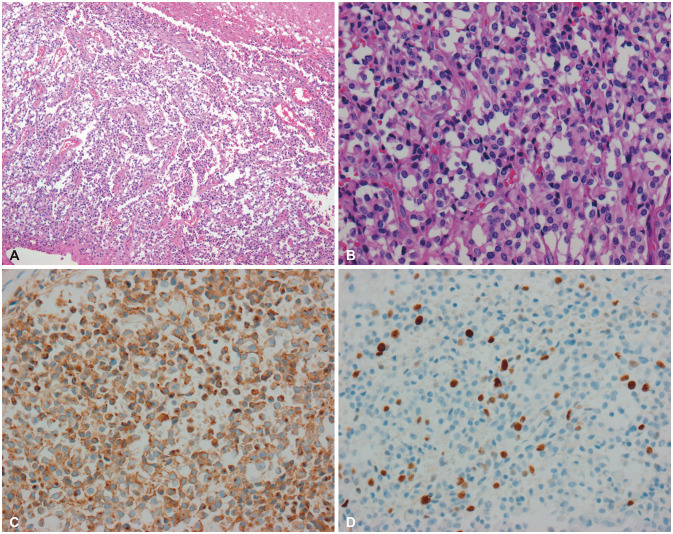
Table 1
Basal characteristics of enrolled patients
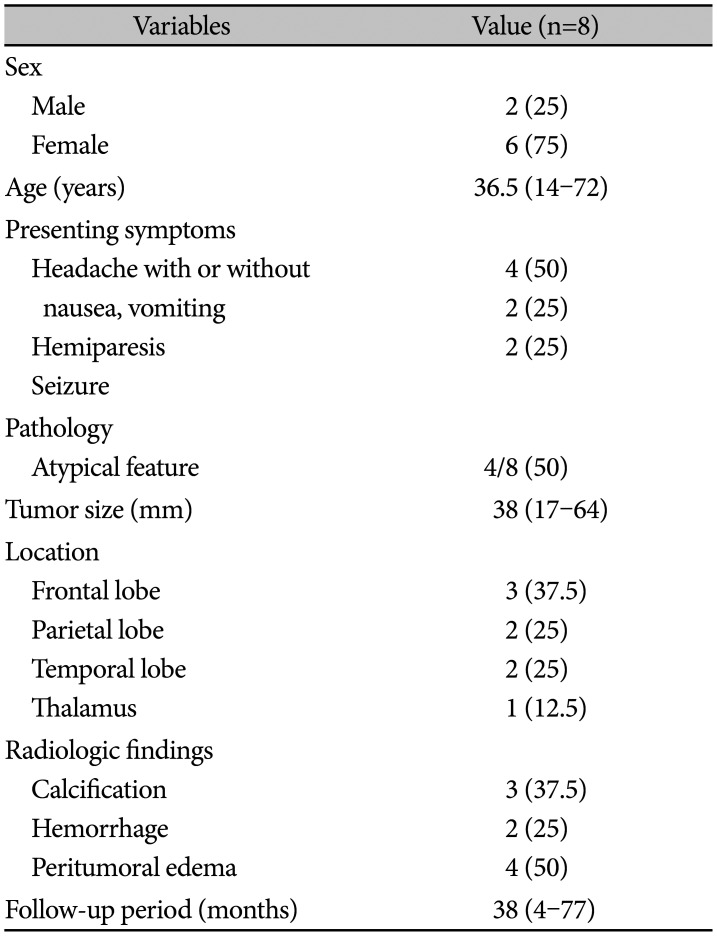
Table 2
Detailed patient characteristics and treatment outcome
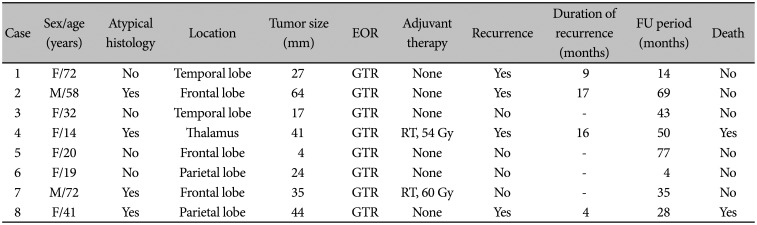
Table 3
Treatment outcomes of EVN
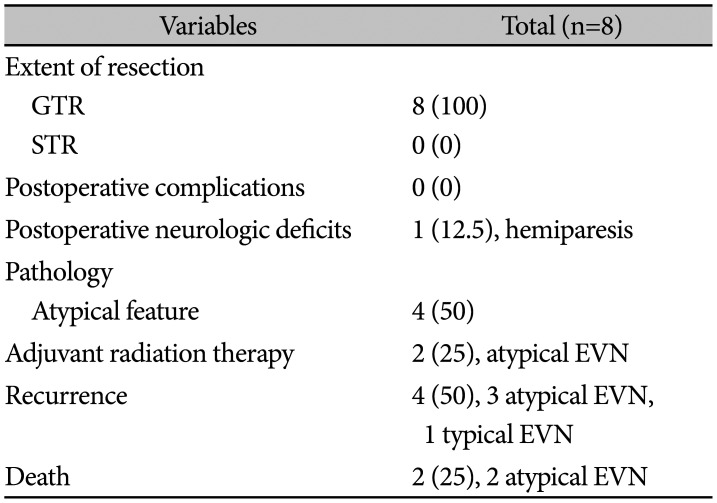
Table 4
Immunohistochemical findings of extraventricular neurocytoma




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download