Abstract
Brainstem gliomas are not common in adults, and the treatment strategies and their outcomes are limited. Immunotherapy is emerging as a promising new modality for the treatment of these gliomas. Here, we report the first case of brainstem glioma treated with a combination of radiotherapy and autologous formalin-fixed tumor vaccine (AFTV). A 32-year-old man presented with left facial numbness and right hemiparesis, and was referred to our department. MRI and open biopsy indicated brainstem glioma, and he was specifically diagnosed with isocitrate dehydrogenase 1-mutant diffuse astrocytoma of WHO grade II. He was treated with stereotactic radiotherapy followed by AFTV three months later. MRI conducted at 42 months after the combination therapy showed a 91% decrease in tumor volume, and the regression was maintained for 5 years. Thus, combination treatment with radiotherapy and immunotherapy may prove to be a promising alternative for the treatment of brainstem glioma.
Brainstem gliomas form a heterogeneous group of tumors that includes diffuse instrinsic pontine glioma, glioblastoma, diffuse astrocytoma, oligodendroglioma, and pilocytic astrocytoma (PA), which are located in the midbrain, pons, and medulla. Brainstem gliomas are not commonly encountered in adults, and they constitute less than 2% of adult gliomas [1]. Based on DNA methylation microarray, Chen et al. [2] proposed four major methylation clusters of brainstem gliomas, namely, histone 3 (H3)-pons, H3-medulla, isocitrate dehydrogenase (IDH), and PA-like, each of which are associated with unique genomic and clinical profiles. Methylation cluster H3-pons and H3-medulla are enriched for H3K27M and G34R/V mutations. H3-pons is consisted of grade III–IV gliomas and represents the most aggressive clinical course in these groups. Methylation cluster IDH occurs in adults and is mainly comprised of WHO grade II gliomas. Methylation cluster IDH dose not represent a more malignant clinical course than H3-pons or H3-medulla.
Typically, patients diagnosed with brainstem gliomas are treated with radiotherapy at 50–55 Gy, using fractions of 1.8–2.0 Gy [1]. Chemotherapy is mainly used as adjuvant treatment, and the most frequent regimen is the Stupp regimen, which involves administration of both concurrent and adjuvant temozolomide [34]. However, the survival rates are still dismal. For example, the average survival in pediatric diffuse intrinsic pontine glioma is uniform across studies at 10–12 months, with survival beyond 3 years being rare; further, adult studies on infiltrating, brainstem gliomas indicate an average survival of 30–40 months, but survival decreases with age [3].
As an alternative treatment strategy, immunotherapy is considered to be potentially beneficial for treatment against untreatable gliomas, and several clinical trials on immunotherapy against glioblastoma have recently been reported [567]. An emerging immunotherapy option is autologous formalin-fixed tumor vaccine (AFTV), which uses autologous formalin-fixed tumor tissue fragments and adjuvants to induce tumor specific immune reactions [8]. Specifically, AFTV involves the induction of activated CD8+ cytotoxic T lymphocytes. In patients with the tumor, tumor associated antigens are presented by antigen presenting cells, but the immune system is suppressed in the tumor-bearing state [9]. However, the tumor tissue and adjuvant delivered by AFTV therapy result in the recovery and activation of cellular immune function [1011]. So far, the efficacy of AFTV for glioblastoma [12] and hepatocellular carcinoma [8] has been evaluated, but its efficacy for brainstem gliomas has not yet been reported.
Here, the authors report the case of a patient with brainstem glioma in whom partial remission was achieved with a combination of radiotherapy and AFTV.
A 32-year-old man with no past medical history was referred to our department for left facial numbness and right hemiparesis. MRI of the head showed hyperintense signals in the pons, midbrain, and left middle cerebellar peduncle on T2 and FLAIR (Fig. 1A-C) images, and the tumor volume was determined to be 23.9 cm3. MRI also demonstrated faint enhancement of the same lesion on T1 images (Fig. 1D). These findings were indicative of brainstem glioma as well as demyelinating diseases, such as acute disseminated encephalomyelitis and multiple sclerosis, which were considered in the differential diagnosis. We performed an open biopsy in order to obtain a definitive diagnosis. Microscopic evaluation of the biopsy sample revealed an infiltrative, diffuse growth pattern of glioma cells (Fig. 2A), and the immunohistochemistry (IHC) findings for GFAP, p53, and IDH1R132H were positive (Fig. 2B and C). The IHC findings for H3K27M were negative (Fig. 2E), and the Ki-67/MIB-1 index was 2%–3% (Fig. 2D). Eventually, he was diagnosed with “diffuse astrocytoma, IDH mutant.”
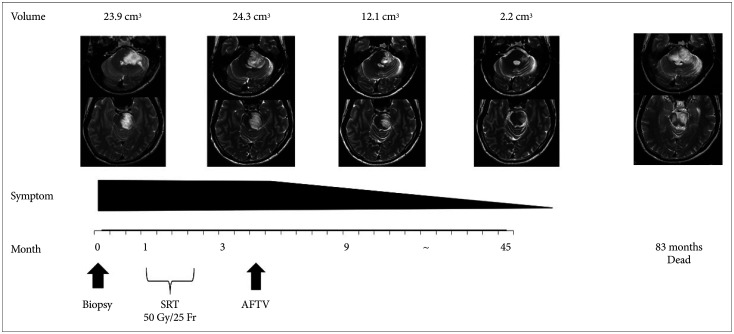
The patient received a total dose of 50 Gy of stereotactic radiotherapy that was delivered in 25 fractions of 2 Gy each, and his neurologic deficits resolved. Three months after radiotherapy, the patient expressed interest in undergoing AFTV at another clinic. Therefore, we introduced him to a clinic for immunotherapy with AFTV. The AFTV treatment usually comprises three courses of vaccination performed at 2-week intervals, but, in this case, only one course of vaccination was administered because very little tumor tissue was removed. The treatment consisted of five local intradermal injections of 0.2 mL AFTV per site in the upper arm. MRI performed 6 months after the open biopsy showed regression of the tumor. Further, MRI performed 42 months after AFTV demonstrated significant tumor reduction: The tumor volume was 2.2 cm3, and it exhibited a 91% reduction compared with the initial MRI. The symptoms of the patient resolved after AFTV. This was considered as a partial response based on immunotherapy Response Assessment in Neuro-Oncology criteria [13]. Further, the radiographic responses indicated clinical improvement (Fig. 3). Regression of the tumor was maintained for 60 months.
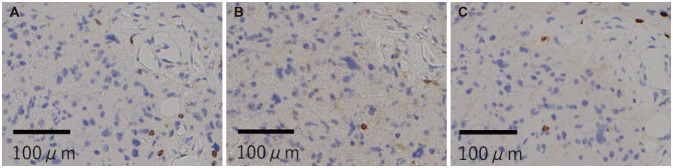
MRI performed 66 months after the open biopsy showed progression disease and the symptoms worsened, so temozolomide was administered. However, the tumor regrowth was not inhibited, and a second open biopsy was performed. The biopsy results were, again, indicative of diffuse astrocytoma, IDH mutant. IHC analysis of CD3, CD4, and CD8 (Fig. 4) demonstrated poor infiltration of immune cells. The neurologic findings were unchanged. Based on these findings, three additional courses of AFTV treatment were administered at 2-week intervals. Despite the additional AFTV administration, MRI demonstrated regrowth of the tumor, and the facial palsy and hemiparesis got worse. And, 83 months after the first open biopsy, the patient died because of disease progression.
In the patient case report, we describe a case of brainstem glioma that was diagnosed and classified as “diffuse astrocytoma, IDH mutant (WHO grade II),” based on the detection of an IDH mutation and wild-type H3.
Treatment strategies against brainstem gliomas are limited, because surgery is often not possible and even biopsies are challenging and associated with a higher risk of complications. For adults with brainstem glioma, the standard therapy is involved-field radiotherapy with a total dose of 54 Gy delivered in 1.8 to 2.0 Gy [14]. Furthermore, Schulz-Ertner et al. [15] reported the efficacy of fractionated stereotactic radiotherapy (54 Gy delivered in 1.8 Gy fractions) against brainstem gliomas, but the median overall survival was 40 months. This clinical trial included 41 patients with brainstem gliomas; histology revealed low grade gliomas in 30 patients and tumors have not been classified in 10 patients. Chemotherapy is also possibly another potential treatment, but the available literature is limited. Reyes-Botero et al. [16] demonstrated that patients with recurrent low grade diffuse brainstem glioma were treated with temozolomide after radiotherapy. Median OS was 14.4 months. Mellinghoff et al. [17] reported the potential use of IDH1 inhibitors against the IDH mutant gliomas. In this clinical trial, ivosidenib was associated with a favorable safety profile, prolonged disease control, and reduced growth of nonenhancing tumor.
Given the less-than-satisfactory outcomes of the current radiotherapy regimens, immunotherapy could be a promising alternative for brainstem glioma in adults. In fact, AFTV, an emerging immunotherapy modality, has shown efficacy for the treatment of glioblastomas [12] and hepatocellular carcinoma [8]. Further, Ishikawa et al. [12] reported a phase I/IIa trial of fractionated radiotherapy, temozolomide, and AFTV for newly diagnosed glioblastoma, in which the combination treatment was found to be well tolerated and resulted in favorable progression-free survival and overall survival. Similarly, in the present case, the brainstem glioma responded well to a combination of stereotactic radiotherapy delivered in doses of 2.0 Gy (total dose=50 Gy) and AFTV. In this case, the abscopal effect of the immune-mediated anti-tumor mechanism of radiotherapy might have triggered the regression of metastatic tumors that were distant from the irradiated field [18]. As AFTV was administered after radiotherapy, the immune system had possibly acquired the ability to respond specifically to the tumor antigen and similar abscopal mechanisms might have occurred in the residual tumor lesion. At 42 months after the combination therapy, a 91% decrease in tumor volume was observed, and tumor regression was maintained for 60 months after combined therapy. These findings indicate that the combination of radiotherapy and AFTV was successful in achieving partial regression in this case of brainstem glioma and may be a promising therapeutic option for similar cases in the future.
There are several limitations of the findings in this case report. First, we could not demonstrate whether immune cells had infiltrated the tumor when regression of the tumor occurred. If the presence of infiltrating immune cells can be proven, it would shed light on the abscopal mechanisms of combination treatment with radiotherapy and immunotherapy against brainstem glioma and its possible benefits.
In conclusion, combination treatment of radiotherapy and AFTV might be a promising treatment for brainstem glioma in adults.
Acknowledgments
We thank the referring physicians, our institutional neuroradiologists and pathologists.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Reyes-Botero G, Mokhtari K, Martin-Duverneuil N, Delattre JY, Laigle-Donadey F. Adult brainstem gliomas. Oncologist. 2012; 17:388–397. PMID: 22382458.

2. Chen LH, Pan C, Diplas BH, Xu C, Hansen LJ, Wu Y, et al. The integrated genomic and epigenomic landscape of brainstem glioma. Nat Commun. 2020; 11:3077. PMID: 32555164.

3. Theeler BJ, Ellezam B, Melguizo-Gavilanes I, de Groot JF, Mahajan A, Aldape KD, et al. Adult brainstem gliomas: correlation of clinical and molecular features. J Neurol Sci. 2015; 353:92–97. PMID: 25934342.

4. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996. PMID: 15758009.

5. Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019; 25:477–486. PMID: 30742122.

6. Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020; 6:1003–1010. PMID: 32437507.

7. Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, et al. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018; 16:142. PMID: 29843811.
8. Ohno T. Autologous cancer vaccine: a novel formulation. Microbiol Immunol. 2003; 47:255–263. PMID: 12801062.

9. Zou JP, Shimizu J, Ikegame K, Yamamoto N, Ono S, Fujiwara H, et al. Tumor-bearing mice exhibit a progressive increase in tumor antigen-presenting cell function and a reciprocal decrease in tumor antigen-responsive CD4+ T cell activity. J Immunol. 1992; 148:648–655. PMID: 1345922.

10. Zou JP, Nagata T, Yamamoto N, Ono S, Fujiwara H, Hamaoka T. Recovery of antitumor CD4+ T cell responsiveness, suppressed in the tumor-bearing state, by release from tumor burden. J Cancer Res Clin Oncol. 1994; 120:279–285. PMID: 7907334.

11. Ishikawa E, Yamamoto T, Matsumura A. Prospect of immunotherapy for glioblastoma: tumor vaccine, immune checkpoint inhibitors and combination therapy. Neurol Med Chir (Tokyo). 2017; 57:321–330. PMID: 28539528.

12. Ishikawa E, Muragaki Y, Yamamoto T, Maruyama T, Tsuboi K, Ikuta S, et al. Phase I/IIa trial of fractionated radiotherapy, temozolomide, and autologous formalin-fixed tumor vaccine for newly diagnosed glioblastoma. J Neurosurg. 2014; 121:543–553. PMID: 24995786.

13. Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015; 16:e534–e542. PMID: 26545842.

15. Schulz-Ertner D, Debus J, Lohr F, Frank C, Hoss A, Wannenmacher M. Fractionated stereotactic conformal radiation therapy of brain stem gliomas: outcome and prognostic factors. Radiother Oncol. 2000; 57:215–223. PMID: 11054526.

16. Reyes-Botero G, Laigle-Donadey F, Mokhtari K, Martin-Duverneuil N, Delattre JY. Temozolomide after radiotherapy in recurrent “low grade” diffuse brainstem glioma in adults. J Neurooncol. 2014; 120:581–586. PMID: 25139026.

17. Mellinghoff IK, Ellingson BM, Touat M, Maher E, De La Fuente MI, Holdhoff M, et al. Ivosidenib in isocitrate dehydrogenase 1-mutated advanced glioma. J Clin Oncol. 2020; 38:3398–3406. PMID: 32530764.
18. Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018; 11:104. PMID: 30115069.

Fig. 1
Scans from MRI of the head conducted on admission. A-C: T2-weighted images showed hyperintense lesions in the pons, midbrain, and left middle cerebellar peduncle. D: Contrast-enhanced MRI demonstrated mild enhancement of the lesion.
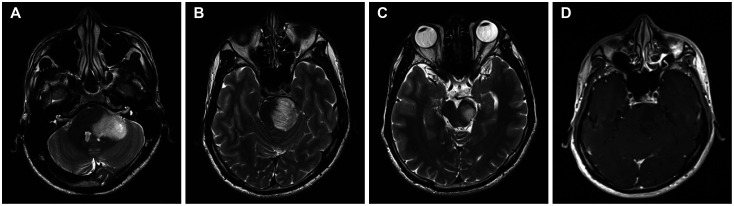
Fig. 2
Histopathological and immunohistochemistry findings for the first open biopsy sample. A: Histopathological examination of the tumor with hematoxylin and eosin staining revealed an infiltrative, diffuse growth pattern of glioma cells. B and C: Immunohistochemistry staining for mutant IDH1 (B) and p53 (C) showed positive results. D: The Ki-67/MIB-1 index was 2%–3%. E: The staining results for H3K27M were negative.
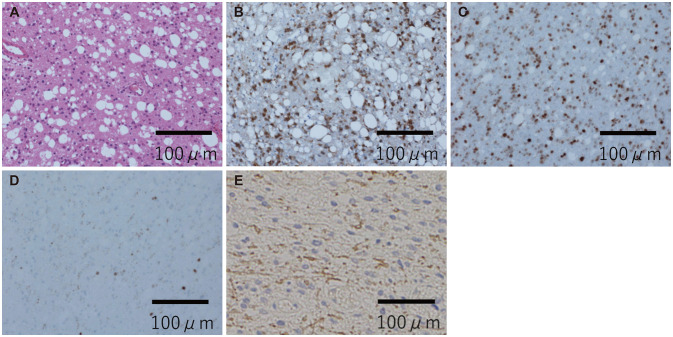
Fig. 3
Clinical course and the corresponding MRI scans. Open biopsy was first performed, and based on the findings, SRT and one course AFTV were performed. Six months after open biopsy, MRI showed regression of the tumor. MRI conducted at 42 months demonstrated significant regression of the tumor. The radiographic response indicated clinical improvement. AFTV, autologous formalin-fixed tumor vaccine; SRT, stereotacic radiotherapy.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download