1. Goyal N, Kakkar A, Singh PK, Sharma MC, Chandra PS, Mahapatra AK, et al. Intracranial teratomas in children: a clinicopathological study. Childs Nerv Syst. 2013; 29:2035–2042. PMID:
23568500.

2. Georgiu C, Opincariu I, Cebotaru CL, Mirescu ŞC, Stănoiu BP, Domşa TA, et al. Intracranial immature teratoma with a primitive neuroectodermal malignant transformation - case report and review of the literature. Rom J Morphol Embryol. 2016; 57:1389–1395. PMID:
28174809.
3. Abdelmuhdi AS, Almazam AE, Dissi NA, Albastaki UM, Pierre-Jerome C. Intracranial teratoma: imaging, intraoperative, and pathologic features: AIRP best cases in radiologic-pathologic correlation. Radiographics. 2017; 37:1506–1511. PMID:
28898192.

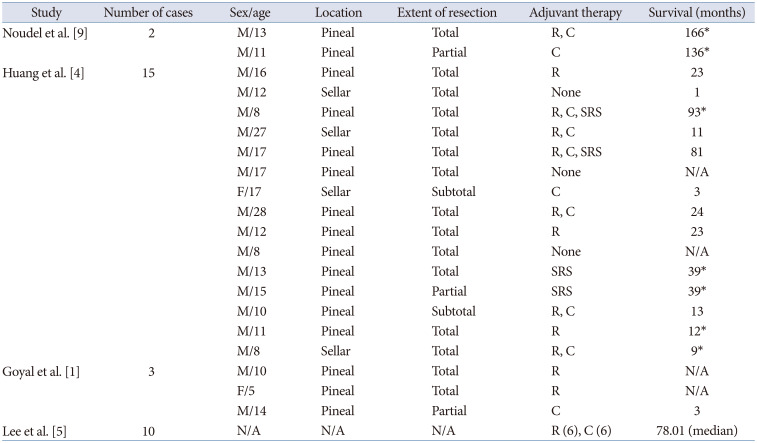
4. Huang X, Zhang R, Zhou LF. Diagnosis and treatment of intracranial immature teratoma. Pediatr Neurosurg. 2009; 45:354–360. PMID:
19907199.

5. Lee YH, Park EK, Park YS, Shim KW, Choi JU, Kim DS. Treatment and outcomes of primary intracranial teratoma. Childs Nerv Syst. 2009; 25:1581–1587. PMID:
19693515.

6. Ogawa K, Toita T, Nakamura K, Uno T, Onishi H, Itami J, et al. Treatment and prognosis of patients with intracranial nongerminomatous malignant germ cell tumors: a multiinstitutional retrospective analysis of 41 patients. Cancer. 2003; 98:369–376. PMID:
12872359.

7. Ferraz ST, Valera ET, Brassesco MS, Santos de Oliveira R, Carlos dos Santos A, Saggioro FP, et al. Intracranial teratoma in children: the role of chromosome 21 trisomy. Neuropathology. 2014; 34:197–200. PMID:
24812702.

8. Liu Z, Lv X, Wang W, An J, Duan F, Feng X, et al. Imaging characteristics of primary intracranial teratoma. Acta Radiol. 2014; 55:874–881. PMID:
24103916.

9. Noudel R, Vinchon M, Dhellemmes P, Litré CF, Rousseaux P. Intracranial teratomas in children: the role and timing of surgical removal. J Neurosurg Pediatr. 2008; 2:331–338. PMID:
18976103.

10. Han JW, Koh KN, Kim JY, Baek HJ, Lee JW, Shim KW, et al. Current trends in management for central nervous system germ cell tumor. Clin Pediatr Hematol Oncol. 2016; 23:17–27.
11. Zhang X, Wang H, Hong F, Xu T, Chen J. “Bones in the medulla oblongata?”— A case report of intracranial teratoma and review of the literature. Front Pediatr. 2021; 9:628265. PMID:
34026683.

12. Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology. 2006; 239:650–664. PMID:
16714456.

13. Beschorner R, Schittenhelm J, Bueltmann E, Ritz R, Meyermann R, Mittelbronn M. Mature cerebellar teratoma in adulthood. Neuropathology. 2009; 29:176–180. PMID:
18627482.
14. Li Q, You C, Zan X, Chen N, Zhou L, Xu J. Mature cystic teratoma (dermoid cyst) in the sylvian fissure: a case report and review of the literature. J Child Neurol. 2012; 27:211–217. PMID:
22190504.

15. Hoffman HJ, Otsubo H, Hendrick EB, Humphreys RP, Drake JM, Becker LE, et al. Intracranial germ-cell tumors in children. J Neurosurg. 1991; 74:545–551. PMID:
1848284.
16. Ziyal IM, Bozkurt G, Bilginer B, Gülsen S, Ozcan OE. Abducens nerve palsy in a patient with a parasagittal meningioma--case report. Neurol Med Chir (Tokyo). 2006; 46:98–100. PMID:
16498221.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download