This article has been
cited by other articles in ScienceCentral.
Abstract
Peripheral scalp T-cell lymphoma is a very rare disease. We report a case of a 22-year-old man who presented an indolent large scalp mass in the right frontal scalp region. The patient’s physical examination demonstrated no palpable mass in the chest, abdomen, and extremities. The brain CT revealed a high-density large scalp mass of the subgaleal layer in the right frontal and a small scalp mass of the subgaleal layer in the left frontal. The brain MRI showed multifocal enhancing masses in the bilateral dura, the subgaleal layer of the scalp, and the skull. The patient underwent removal of the tumor found in the right frontal scalp. The histologic diagnosis was peripheral T-cell lymphoma. Bone marrow aspiration showed the involvement of T-cell lymphoma. The patient received chemotherapy with cyclophosphamide, vincristine, doxorubicin, and prednisolone (CHOP protocol) for 3 cycles. The patient was discharged without neurological deficit. The patient showed no evidence of recurrence 15 months after surgery. We report a rare case of peripheral T-cell lymphoma mimicking benign scalp tumors.
Keywords: Lymphoma, Mass, Scalp
INTRODUCTION
Peripheral T-cell lymphoma is a rare aggressive lymphoma, that mainly involves extranodal sites including spleen, liver, skin, and intestine [
1]. The tumors are composed of small-medium cells with CD4
–CD8
+ phenotype. Non-Hodgkin’s lymphoma is a broad class of malignant neoplasms originating from B cell progenitors, T cell progenitors, mature B cells, mature T cells, and natural killer cells in rare cases. In addition to the rarity of T-cell lymphomas, cranial vault involvement is extremely rare in primary non-Hodgkin’s lymphomas [
2]. We report a case of peripheral scalp T-cell lymphoma with a favorable clinical course after surgery and chemotherapy.
CASE REPORT
A 22-year-old man with no previous medical or surgical history presented with a large, indolent mass in the right frontal scalp and a small mass in the left frontal scalp region. The patient showed no signs of fever, general weakness, hepatomegaly, splenomegaly, other than the palpable masses in both frontal lobes involving the scalp. Non-enhanced CT revealed large scalp mass of the subgaleal layer in the right frontal and a small scalp mass of the subgaleal layer in the left frontal (
Fig. 1). The mass measured 8 cm and showed no evidence of invasion in the skull on CT and MRI. Brain MRI revealed multifocal homogeneously enhancing lesions in the bilateral dura, multiple T2-hyperintense and homogeneously enhancing mass-like lesions involving subgaleal layer of the scalp, left forehead, and left superior orbit, multiple ill-defined enhancing lesions in the skull (
Fig. 2). Under general anesthesia, the patient underwent an
en bloc resection of the mass on the right frontal scalp. The tumor had mildly invaded the skull and was yellow-gray, firm and relatively hypervascular. PET-CT showed mildly hypermetabolic lesions in the lymph nodes in the neck, a diffuse increase of FDG uptake in the bone marrow through the entire body, the right parotid gland, and the anterior mediastinum, the frontal scalp (
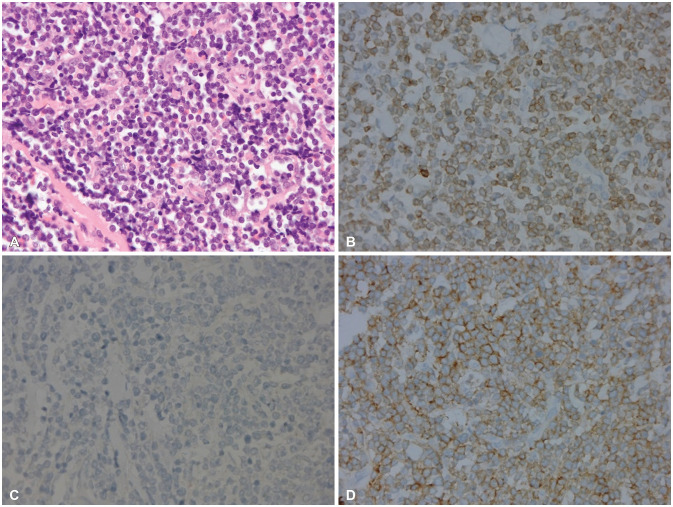
Fig. 3). Bone marrow aspiration showed the involvement of T-cell lymphoma. The pathological diagnosis was peripheral T-cell lymphoma of the scalp. Tumor cells were positive for CD3, negative for CD20, negative for CD4, and positive for CD8. All follicular helper T cell markers (CD10, BCL6, PD-1) were not expressed.
In-situ hybridization for Epstein-Barr virus-encoded RNAs were negative. Ki-67 proliferation index were about 50% to 60% (
Fig. 4).
After the histological diagnosis was confirmed the patient received CHOP chemotherapy (cyclophosphamide, vincristine, doxorubicin, and prednisolone) for 3 cycles. The patient was discharged without neurological deficit and showed no evidence of recurrence 15 months after surgery.
DISCUSSION
Most primary lymphomas of the central nervous system (CNS) are of the large B-cell type. A distinctive variant of the systemic lymphoma with the tendency to involve the angiotrophic lymphoma (intravascular lymphoma) is also predominantly of B-cell lineage [
3]. On the other hand, there are rare cases of peripheral T-cell lymphoma. Peripheral T-cell lymphoma of the CNS with the cytotoxic phenotype are composed of several entities [
1]. These lymphomas represent a heterogenous group of lymphoma, with the majority as the CD4
–CD8
+ T-helper cell immunophenotype, a minority with a CD4
–CD8
+ cytotoxic T cell immunophenotype and double positive or negative in rare cases [
456].
Primary lymphomas of the CNS may vary widely in MRI appearance, including its location, the number of lesions, and looks. It can be found in the superficial cortical layer or the deep periventricular area. It can also be solitary or multiple lesions. Furthermore, it may mimic the image of glioblastoma as a butterfly lesion of the corpus callosum. In contrast, secondary lymphomas preferentially involves the leptomeninges.
In our case, a large high-density scalp mass was demonstrated by a CT scan. The initial symptoms and signs of lymphoma in the skull include a painless scalp lump, which was observed in 90% of patients; headache due to bone destruction or tumor infiltration of meninges was observed in 30%; seizures and focal neurological deficits resulting from infiltration of the cortex presented respectively in 10% and 20% of cases [
789]. The disease may primarily involve the skin, particularly in the head and neck region, and a rapidly growing, often painful mass can be seen to be of single nodal or extranodal site [
789]. Only 15% to 25% of cutaneous lymphomas show extracutaneous manifestations at time of diagnosis [
3]. Microscopically, malignant T cells express CD45 and T cell antigens (CD2), although loss of T-cell antigens may occur. Some cases are positive for the cytotoxic granule proteins granzyme B and perfolin [
45]. Molecular genetic demonstration of T-cell monoclonality can be helpful in distinguishing T-cell lymphoma from T-cell-rich large B-cell lymphoma and inflammation. Their histological and immunophenotypic features may vary, but low-grade appearance is relatively common [
45]. Treatment for newly diagnosed lymphoma consists of induction phase and a remission-consolidation phase. Typically, induction consists of chemotherapy with the objective of achieving a complete response. Once this response/remission is achieved, a different therapy regimen or whole brain radiation therapy is administered to treat lymphoma. We consulted the hematooncology doctor for chemotherapy. Anthracycline-containing regimens, namely CHOP, nowadays represent the standard first-line treatment for peripheral T-cell lymphoma. The overall response rate was 91%, the 2-year overall survival was 87% [
10]. As per such standards, the patient received CHOP chemothereapy. Peripheral T-cell scalp lymphoma is an extremely rare disease. Despite its rarity, it should not be dismissed in the differential diagnosis of scalp masses. Occurrences of this entity and the variability of follow-up, the combination of surgery, radiotherapy, and chemotherapy seem to offer better outcomes. It is important, despite the similarity in the images, to acknowledge the wide range of diagnoses of a mass and to not overlook the low number of cases of peripheral T-cell lymphoma.
Availability of Data and Material
The datasets generated or analysed during the study are avaialable from the corresponding author on reasonable request.
References
1. Berti E, Tomasini D, Vermeer MH, Meijer CJ, Alessi E, Willemze R. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. A distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999; 155:483–492. PMID:
10433941.

2. Duyndam DA, Biesma DH, van Heesewijk JP. Primary non-Hodgkin’s lymphoma of the cranial vault; MRI features before and after treatment. Clin Radiol. 2002; 57:948–950. PMID:
12413922.

3. Batchelor TT. Primary central nervous sysytem lymphomas. Richard Winn H, editor. Youmans and Winn neurological surgery. 7th ed. Philadelphia: Elsevier;2017. p. 1085–1090.
4. Ikumi N, Fujita H, Terui T, Takahashi H, Miura K, Hatta Y, et al. Aggressive CD4
–CD8
–CD45RA
+CCR10
– primary cutaneous peripheral T-cell lymphoma, not otherwise specified: a case report. Acta Derm Venereol. 2019; 99:1176–1177. PMID:
31502655.
5. Lim T, Kim SJ, Kim K, Lee JI, Lim DH, Lee DJ, et al. Primary CNS lymphoma other than DLBCL: a descriptive analysis of clinical features and treatment outcomes. Ann Hematol. 2011; 90:1391–1398. PMID:
21479535.

6. Mongia S, Shukla D, Devi BI, Reddy TV. Primary cranial vault non-Hodgkin’s lymphoma. Neurol India. 2003; 51:293–294.
7. El Asri AC, Akhaddar A, Baallal H, Boulahroud O, Mandour C, Chahdi H, et al. Primary lymphoma of the cranial vault: case report and a systematic review of the literature. Acta Neurochir (Wien). 2012; 154:257–265. PMID:
21842209.

8. Kantarci M, Erdem T, Alper F, Gundogdu C, Okur A, Aktas A. Imaging characteristics of diffuse primary cutaneous B-cell lymphoma of the cranial vault with orbital and brain invasion. AJNR Am J Neuroradiol. 2003; 24:1324–1326. PMID:
12917120.
9. Burg G, Dummer R, Kerl H. Classification of cutaneous lymphomas. Dermatol Clin. 1994; 12:213–217. PMID:
8045033.

10. da Rocha AJ, da Rocha TM, da Silva CJ, Paes RP, Bruniera P, Chiattone CS. Cranial vault lymphoma: a systematic review of five patients. J Neurooncol. 2010; 100:9–15. PMID:
20146083.
Fig. 1
CT shows enlarged frontal large scalp mass. A: Axial view non-contrast. B: Coronal view non-contrast.
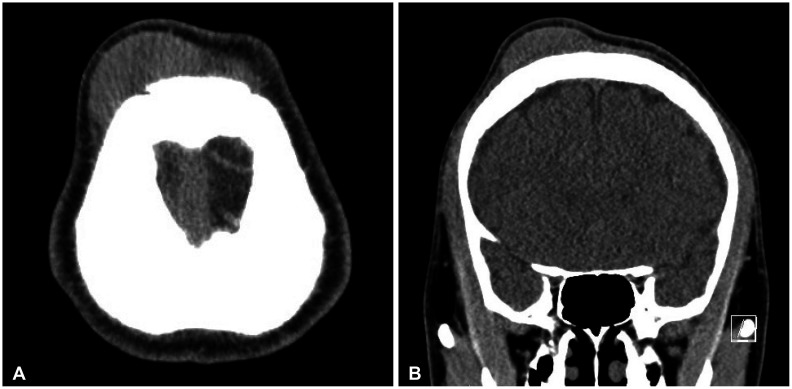
Fig. 2
MRI demonstrating multifocal homogeneously enhancing lesions in the bilateral dura (A) and multiple T2-hyperintense and homogeneously enhancing mass-like lesions involving subgaleal layer of the scalp, left forehead, and left superior orbit, multiple ill-defined enhancing lesions in the skull (B).
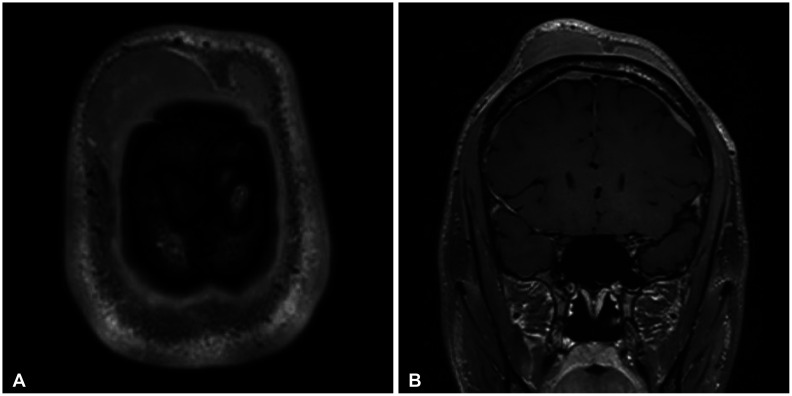
Fig. 3
PET-CT shows mildly hypermetabolic lesions in the lymph nodes in the neck (A) and a diffuse increase of FDG uptake in the bone marrow through the entire body, the right parotid gland, and the anterior mediastinum, the frontal scalp (B).

Fig. 4
Histopatholgy shows peripheral T-cell lymphoma. A: Diffuse infiltrates of medium-sized lymphoid cells with irregular nuclei (H&E, ×400). Tumor cells were positive for CD3 (B, ×400), negative for CD20 (C, ×400), and positive for CD8 (D, ×400). All follicular helper T cell markers (CD10, BCL6, PD-1) were not expressed.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download