INTRODUCTION
MATERIALS AND METHODS
Animals
Isolation and selective culture of SV-ECs
Flow cytometry analysis
Culture and treatment of SV-ECs
Immunofluorescence
Polymerase chain reaction
Apoptosis analysis
β-galactosidase staining
Cell viability assay
High resolution mass spectrometry
Isolation and culture of primary peritoneal Mφ
Enzyme linked immunosorbent assay
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses
Statistical analyses
RESULTS
Establishing a serum-free culture method for SV-ECs
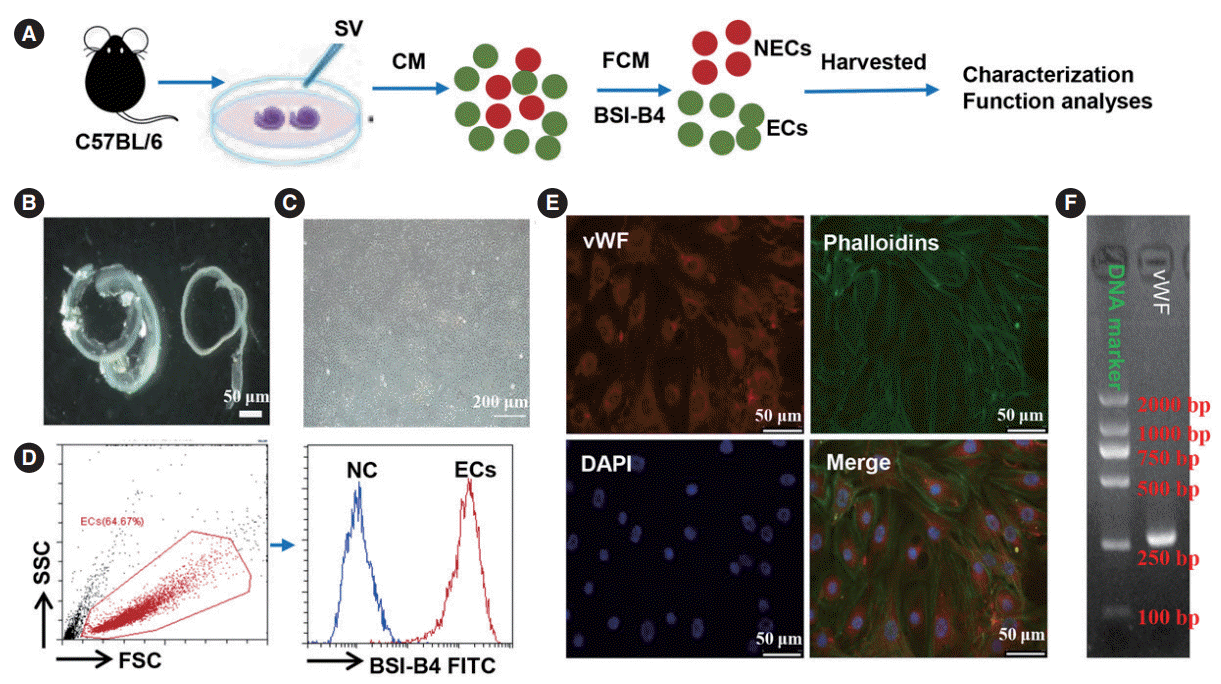 | Fig. 1.Isolation and characterization of stria vascularis endothelial cells (SV-ECs). (A) Schematic diagram illustrating the procedure of isolating ECs. (B, C) The cochlear lateral wall was fully isolated from the cochlea and placed in fresh ice-cold perilymph solution (B), and cochlear ECs were selectively cultured in EC growth medium (C). (D) ECs were characterized by flow cytometry analysis for cell purity. (E) Immunofluorescent images of ECs probed for phalloidins (green), von Willebrand factor (vWF; red), and nuclei (blue). (F) Polymerase chain reaction gene analysis of an EC marker (vWF). CM, conditioned medium; FCM, flow cytometry; NEC, non-endothelial cell; SSC, side scatter; FSC, forward scatter; FITC, fluorescein isothiocyanate; BSI-B4, isolectin B4; DAPI, 4´,6-diamidino-2-phenylindole. |
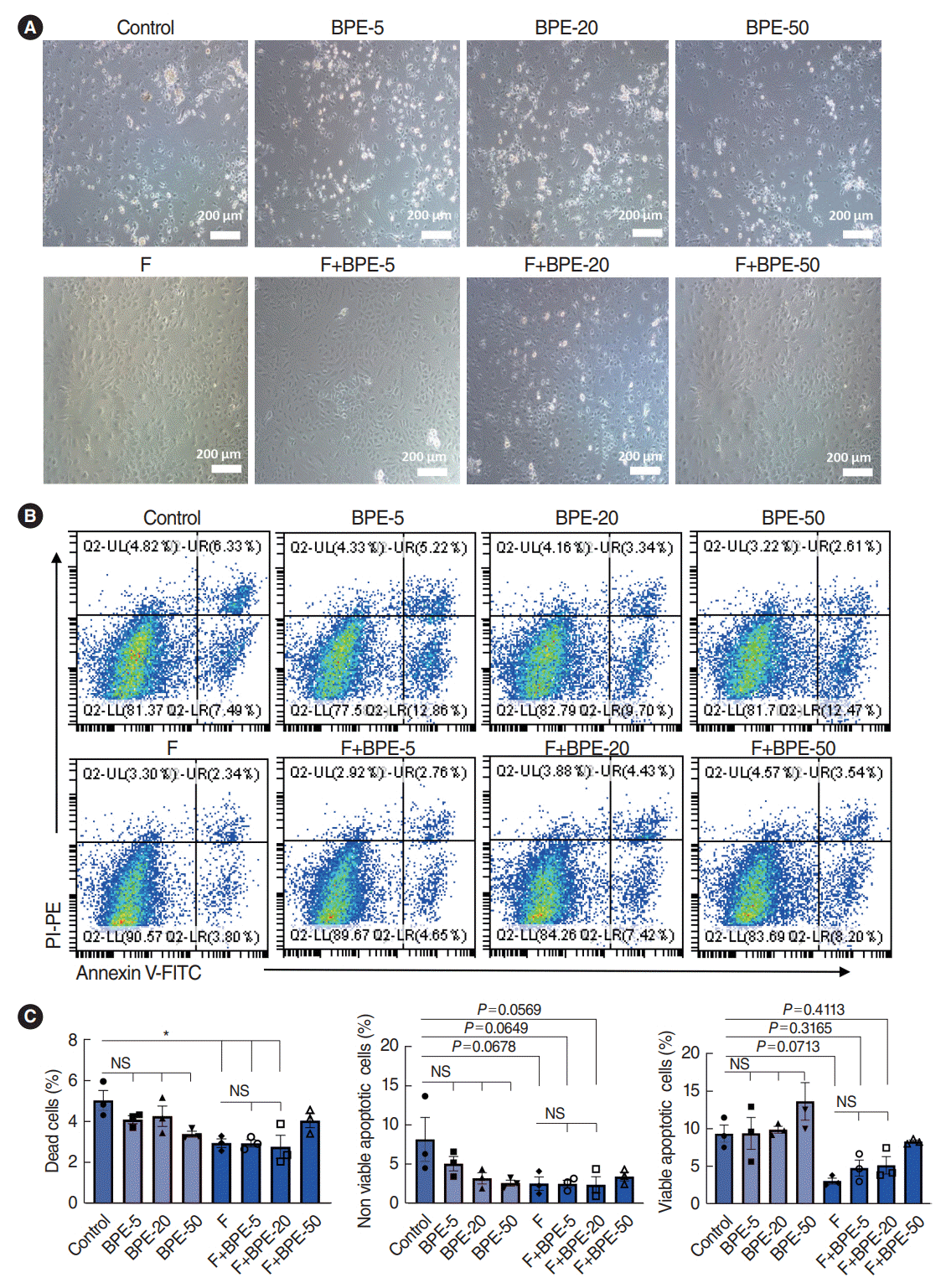 | Fig. 2.Fibronectin maintained the viability of stria vascularis endothelial cells (SV-ECs) in serum-free culture medium. (A) Fibronectin and bovine pituitary extract (BPE) were selected to explore the optimum conditions for serum-free culture of SV-ECs. SV-ECs were treated with different concentrations of BPE in serum-free culture medium for 24 hours in the presence (upper) or absence (lower) of fibronectin. Bright-field microscopy showed that the SV-ECs were in a good condition in the serum-free culture medium with fibronectin-precoated plates, and BPE showed no significant effects on reducing the apoptosis of SV-ECs in the serum-free medium. (B, C) Flow cytometry analyses suggested that the apoptosis rate of SV-ECs in the serum-free culture medium was significantly reduced among SV-ECs cultured in fibronectin-precoated plates. F, fibronectin; UL, upper left; UR, upper right; LL, lower left; LR, lower right; FITC, fluorescein Isothiocyanate; PI, propidium iodide; PE, P-phycoerythrin; NS, not significant. *P<0.05 according to one-way analysis of variance for multiple comparisons. |
Fibronectin reduced the death rate of ECs induced by H2O2 in vitro
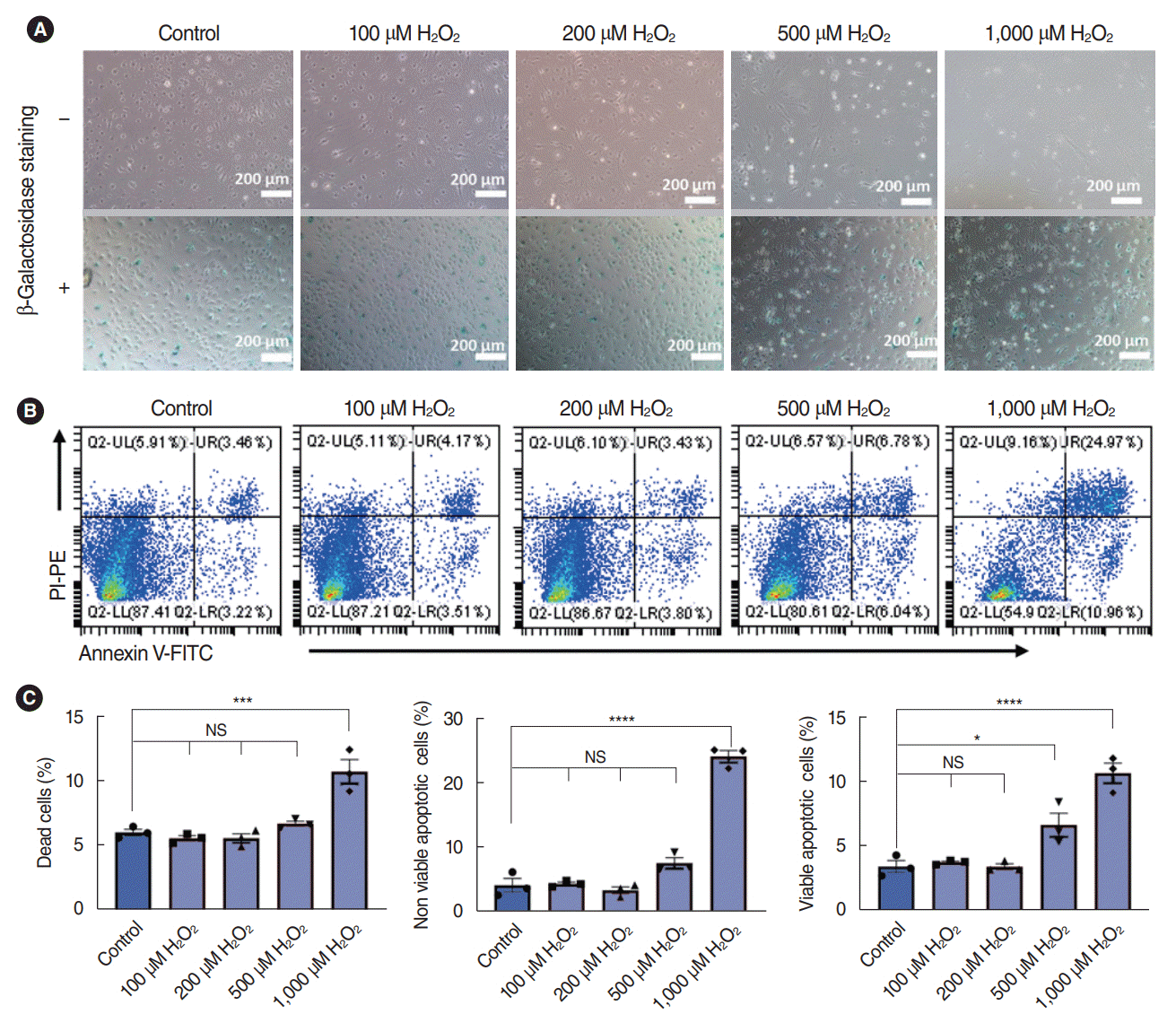 | Fig. 3.Dose-dependent effects of H2O2 on stria vascularis endothelial cells (SV-ECs) cultured with complete culture medium (CCM). (A) Bright-field microscopy with (lower) or without (upper) β-galactosidase staining for ECs pretreated with various concentrations of H2O2 for 2 hours in CCM. The SV-ECs were stimulated with 100 μM, 200 μM, 500 μM, and 1,000 μM H2O2 for 2 hours, and SV-ECs started to detach from the plate (upper) and become significantly senescent (lower) in response to stimulation by 1,000 μM H2O2 compared to the control cells. (B, C) Apoptotic analysis for ECs pretreated with various concentrations of H2O2 for 2 hours in CCM. Values are presented as mean±standard error of the mean (n=3). PI, propidium iodide; PE, P-phycoerythrin; FITC, fluorescein Isothiocyanate; FITC, fluorescein Isothiocyanate; UL, upper left; UR, upper right; LL, lower left; LR, lower right; NS, not significant. *P<0.05, ***P<0.001, ****P<0.0001 according to one-way analysis of variance. |
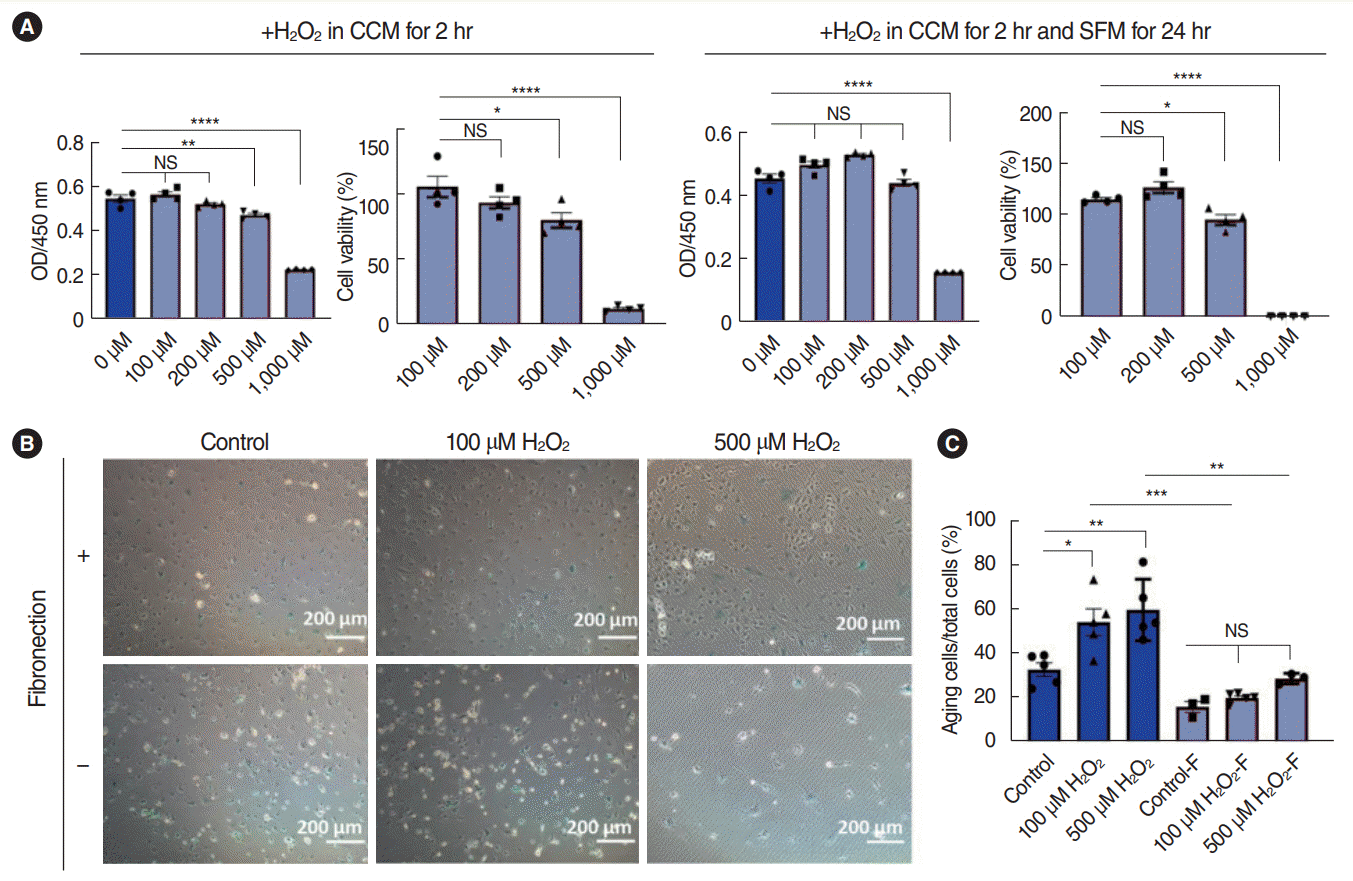 | Fig. 4.Dose-dependent effects of H2O2 on stria vascularis endothelial cells (SV-ECs) cultured in serum-free medium. (A) Cell viability assay for ECs pretreated with various concentrations of H2O2 for 2 hours, followed by incubation with serum-free medium for 24 hours in fibronectin-precoated cell culture plates. (B, C) Bright-field microscopy for β-galactosidase staining of ECs pretreated with 100 μM or 500 μM H2O2 for 2 hours, followed by incubation with serum-free medium for 24 hours in cell culture plates with or without precoating of fibronectin. Values are presented as mean±standard error of the mean (n=3). CCM, complete culture medium; SFM, serum-free culture medium; NS, not significant. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 according to one-way analysis of variance. |
Secretome changes of SV-ECs induced by oxidative stress
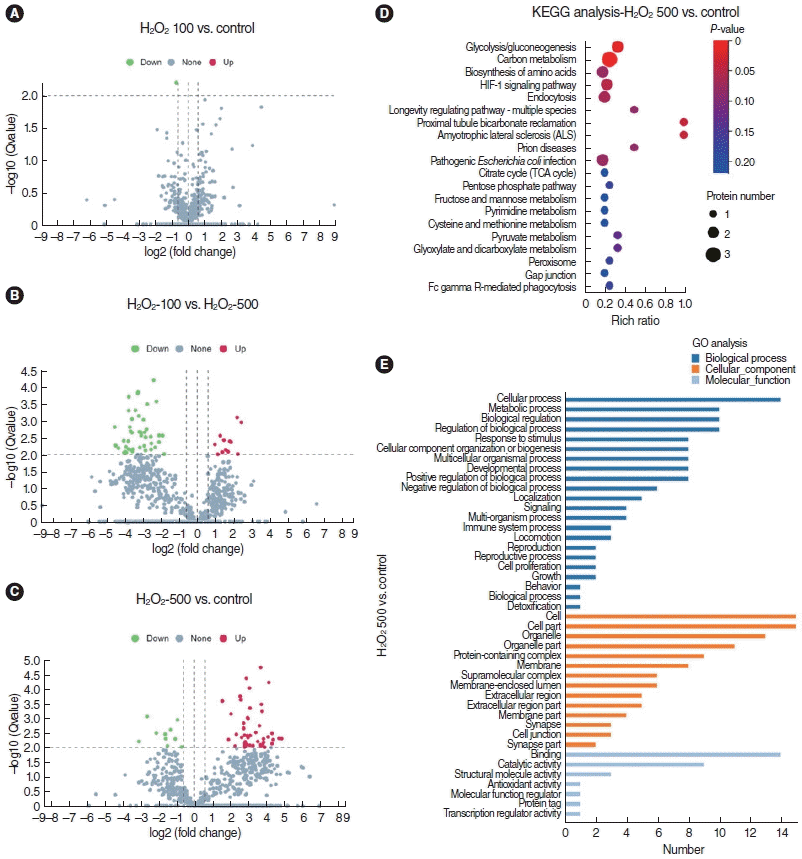 | Fig. 5.LC-MS/MS analysis of supernatant of stria vascularis endothelial cells (SV-ECs) with H2O2. (A-C) Volcano plots for the differential expression analysis of the secretome released by ECs treated with or without H2O2 as indicated. (D, E) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis (D) and Gene Ontology (GO) analysis (E) showed the function of the differential secretome between control cells and ECs treated with 500 μM H2O2. |
Effect of the SV-ECs’ secretome on Mφ
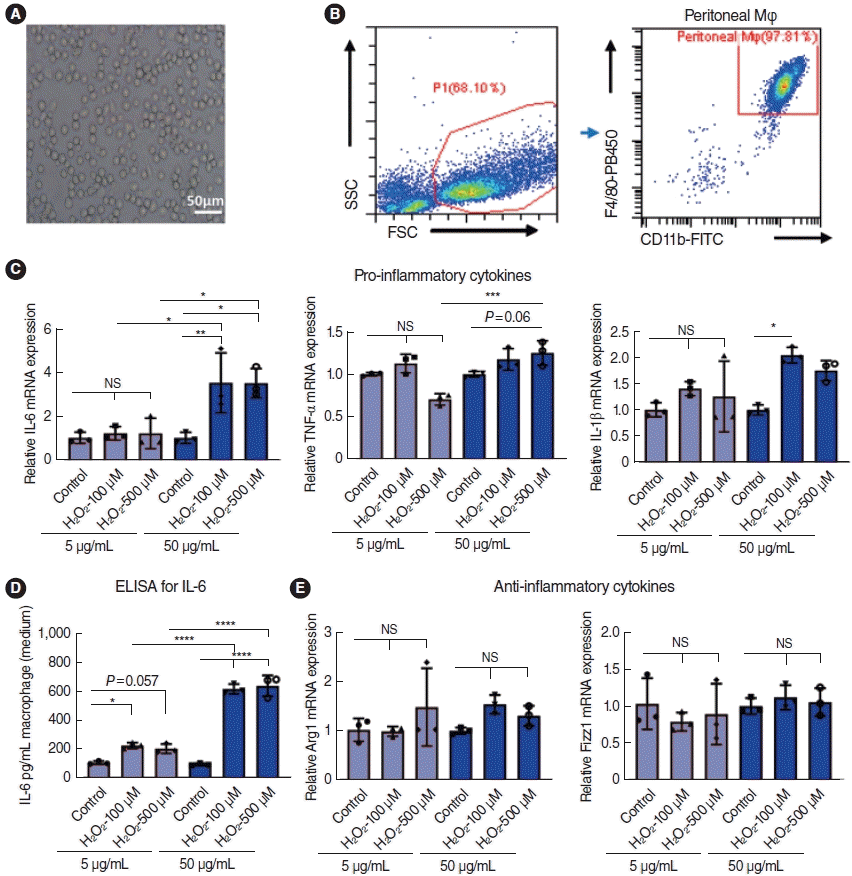 | Fig. 6.Effects of the secretome of stria vascularis endothelial cells (SV-ECs) stimulated with or without H2O2 on peritoneal macrophages (Mφ). (A) Bright-field microscopy of Mφ harvested from the peritoneal cavity. (B) Peritoneal Mφ were characterized by flow cytometry analysis. (C, D) The levels of pro-inflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor (TNF)-α and IL-1β, were increased in the secretomes of SV-ECs stimulated by H2O2 in a dose-dependent manner, as demonstrated by polymerase chain reaction (C) and enzyme-linked immunosorbent assay (ELISA) (D). (E) The secretomes of SV-ECs stimulated by H2O2 showed no effect on levels of anti-inflammatory cytokines, such as Arg1 and Fizz1. SSC, side scatter; FSC, forward scatter; NS, not significant. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 according to one-way analysis of variance. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download