Abstract
Objectives.
When performing middle ear operations, such as ossiculoplasty or stapes surgery, patients and surgeons expect an improvement in air conduction (AC) hearing, but generally not in bone conduction (BC). However, BC improvement has often been observed after surgery, and the present study investigated this phenomenon.
Methods.
We reviewed the preoperative and postoperative surgical outcomes of 583 patients who underwent middle ear surgery. BC improvement was defined as a BC threshold decrease of >15 dB at two or more frequencies. Subjects in group A underwent staged ossiculoplasty after canal wall up mastoidectomy (CWUM), group B underwent staged ossiculoplasty after canal wall down mastoidectomy (CWDM), group C underwent ossiculoplasty only (thus, they had no prior history of CWUM or CWDM), and group D received stapes surgery. We created a hypothetical circuit model to explain this phenomenon.
Results.
BC improvement was detected in 12.8% of group A, 9.1% of group B, and 8.5% of group C. The improvement was more pronounced in group D (27.0%). A larger gain in AC hearing was weakly correlated with greater BC improvement (Pearson’s r=0.395 in group A, P<0.001; r=0.375 in group B, P<0.001; r=0.296 in group C, P<0.001; r=0.422 in group D, P=0.009). Notably, patients with otosclerosis even experienced postoperative BC improvements as large as 10.0 dB, from a mean value of 30.3 dB (standard error [SE], 3.2) preoperatively to 20.3 dB (SE, 3.2) postoperatively, at 1,000 Hz, as well as an improvement of 9.2 dB at 2,000 Hz, from 37.8 dB (SE, 2.6) to 28.6 dB (SE, 3.1).
Pure-tone audiometry, which is the most important test for evaluating hearing loss, measures both air conduction (AC) and bone conduction (BC) thresholds. The AC threshold is a response to sound delivered via a headphone. The AC threshold reflects the functional status of all structures in the sound energy transmission pathway. The thresholds increase (AC hearing decreases) if an abnormality is present in either the external ear (the auditory canal), the middle ear (the tympanic membrane and ossicles), or the inner ear (the cochlea). The BC threshold is measured by placing a bone oscillator on the mastoid process. The oscillator changes the vibration of sound energy, provoking skull motion; this motion is directly transmitted to the cochlea. As BC bypasses the external and middle ear structures, the BC threshold increases (BC hearing decreases) when the inner ear is injured [1].
During AC, sound energy is transferred only through the oval window (OW), whereas BC uses various routes to attain the cochlea. Skull vibration is the most important route, but paths through skin, soft tissue, and fluid also contribute to BC hearing [2,3]. Although the paths to the cochlea differ, both AC and BC ultimately cause the same basilar membrane movements, stimulating the same sensory cells that recognize sound [4-8].
In clinical practice, a decrease in BC hearing (an increased BC threshold) is regarded as a sensorineural impairment (a loss of cochlear sensory cells). Therefore, BC hearing improvement is generally not expected after middle ear surgery. However, such improvement is not uncommon [9-15]. We perform middle ear surgery (ossiculoplasty and stapes surgery) principally to improve AC, but we have observed BC hearing improvement (sometimes >15 dB) after surgery. In the present study, we explored the extent of this improvement, the mechanism involved, and finally how to clinically use this BC hearing improvement phenomenon.
We retrospectively reviewed the data of all patients (583 subjects) who underwent middle ear surgeries, either ossiculoplasty or stapes surgery, to improve hearing between January 2012 and December 2020 in the Department of Otolaryngology, Ajou University Hospital, Suwon, Korea. Patients who underwent tympanoplasty (with or without mastoidectomy) to remove either chronic middle ear lesions or cholesteatoma were not included. All surgeries were performed by a single surgeon (YHC). This study was approved by and performed according to the guideline of the Institutional Review Board of Ajou University Hospital (No. AJIRB-MED-MDB-21-183). The informed consent was waived by the Board.
Preoperative pure-tone audiometry was performed within 3 months before surgery. Postoperative hearing was measured at 6 months after surgery. AC hearing thresholds were measured at 125, 250, 500, 1,000, 2,000, 4,000, and 8,000 Hz; and BC thresholds at 250, 500, 1,000, 2,000, and 4,000 Hz. The bone oscillator measuring BC was placed on the mastoid. Masking was routinely performed when testing BC hearing. Given that interaural attenuation was in play, masking was also applied during AC testing when the right and left ears differed in the hearing level.
All subjects were classified into four groups. Patients in group A underwent staged ossiculoplasty after canal wall up mastoidectomy (CWUM); group B patients underwent staged ossiculoplasty after canal wall down mastoidectomy (CWDM); group C patients underwent ossiculoplasty only (thus, there was no prior CWUM or CWDM in them); and group D patients received stapes surgery.
The chi-square test was used to compare categorical data and the independent t-test was used for parametric numerical data. The paired t-test was employed to compare the pre- and postoperative thresholds at each frequency. The numerical values reported as mean (standard error [SE]). All statistical analyses were performed using IBM SPSS Statistics ver. 25.0 (IBM Corp.). P-values<0.05 were considered statistically significant.
Of the 583 subjects, 339 (58.1%) were female, and the sex ratio did not differ significantly among the groups (P=0.227). Groups C and D were younger than the others, largely because these groups included children with congenital ossicular anomalies (Table 1).
About 30% of patients who underwent ossiculoplasty exhibited BC hearing improvements of >10 dB at ≥2 frequencies (29.1% of group A, 29.7% of group B, and 27.9% of group C patients). The improvements were more pronounced in stapes surgery patients (group D), 45.9% of whom showed improvement. Some subjects demonstrated improvements of even >15 dB at the same frequencies (12.8% of group A, 9.1% of group B, 8.5% of group C, and 27.0% of group D patients) (Table 1).
The BC hearing improvements were greater in patients (in all groups) who gained >30 dB of mean AC as an average of each threshold at 500, 1,000, 2,000, and 4,000 Hz (Fig. 1). Weak correlations were evident between the average AC and BC hearing gains at each frequency (Supplementary Table 1). In groups A to C, the highest correlations were apparent at 1,000 Hz, with Pearson’s r coefficients of 0.395 (P<0.001) for group A, 0.375 (P<0.001) for group B, and 0.296 (P<0.001) for group C; the correlation was highest at 4,000 Hz for group D (r=0.422, P=0.009) (Supplementary Table 1, Fig. 2). The average AC and BC hearing improvements of all subjects are described in Supplementary Fig. 1.
BC hearing improvements were more obvious in patients who underwent stapes surgery to treat otosclerosis (Fig. 3). Eighteen patients in group D were diagnosed with hearing loss caused by otosclerosis; their BCs improved by about 10 dB at both 1,000 and 2,000 Hz. At 1,000 Hz, BC hearing improved from 30.3 dB (SE, 3.2) to 20.3 dB (SE, 3.2), and at 2,000 Hz, an improvement was observed from 37.8 dB (SE, 2.6) to 28.6 dB (SE, 3.1). However, patients with other diagnoses (such as congenital stapedial fixation) prior to stapes surgery exhibited an improvement of 8.4 dB from 31.3 (SE, 3.7) to 22.9 dB (SE, 4.5), only at 2,000 Hz.
It is not unusual to find that BC hearing improves after middle ear surgery [9-16]. Based on our work and a similar study, this improvement seems to be more common after stapes surgery [10]. Stenfelt summarized and suggested a model of the pathways contributing to BC [17]. Although the skull bone is the principal contributor to bone-conducted energy transmission, such energy also imposes sound pressure on the external auditory canal and inertial forces on the tympanic membrane and ossicles [3,6,18]. This suggests that BC can be improved when AC is enhanced by middle ear surgery. Figs. 1 and 2 show that a larger AC hearing gain was associated with better BC hearing gain.
However, this may not be the only mechanism by which BC hearing improves. Although the AC and BC hearing improvements were associated, the correlations were weak. Moreover, in group D, unlike other groups, significant improvements in BC hearing were evident even when the postoperative AC hearing gains were ≤30 dB (Supplementary Fig. 2). Therefore, other factors likely improved the ossicular inertia after stapes surgery. Thus, we sought another mechanism, and were guided by the “third window” theory [19-22]. This theory has been used principally to explain improved BC hearing in patients with superior semicircular canal dehiscence (SSCD) [19,20,23]. In such patients, the BC thresholds are sometimes very low at some frequencies, and BC hearing thus appears to be good. However, after surgically plugging the superior semicircular canal, the thresholds normalize [19,24,25]. The normal inner ear contains two anatomical windows: the OW and the round window (RW). In an SSCD patient, dehiscence of the superior semicircular canal creates the third window. This increases the sensitivity of bone-conducted sound, and BC hearing improves [23]. In summary, as the number of windows increases, BC hearing improves, and as the number decreases, it falls.
We applied this concept to otosclerosis. Early otosclerotic lesions arise in the anterior OW, and progress gradually around this window, finally causing stapedial fixation [26,27]. A sclerotic OW does not function as a window. The patient now has only one functional window. As the number of windows falls, BC hearing diminishes and the BC thresholds rise. However, after stapes footplate mobilization by surgery, the OW gain functions as a window (the patient now has two windows), improving BC hearing. Indeed, our otosclerosis patients exhibited striking BC hearing improvements (Fig. 3A). This concept has been used previously. Bae et al. [22] suggested that if otosclerosis proceeded further, even to the RW (“zero windows” preoperatively), although surgery was successful (only yielding a “single window” postoperatively), the surgical outcomes were likely to be worse than those of cases with intact RWs (“two windows” postoperatively).
We used circuit diagrams to prove our hypothesis (Fig. 4A-C). In the diagram, the upper regions represent the scala vestibuli and the lower regions the scala tympani. The BC model of Rosowski et al. places four impedances in the circuit: in the OW (ROW), the scala vestibuli (RSV), the scala tympani (RST), and the RW (RRW) [23]. The current is the flow of perilymph in the cochlea (arbitrarily assumed to be 2A). AC contributes to this current only via the OW, whereas BC contributes via multiple paths; however, both AC and BC finally produce currents of identical types (that move the basilar membrane based on the pressure difference between the scala vestibuli and scala tympani) [4-8]. The resistances (in Ω) of each impedance were also arbitrarily assigned. However, we considered two points. First, the OW resistance is greater than that of the RW because the OW is stiffer. Secondly, the scala vestibuli impedance is known to be greater than that of the scala tympani [17,28]. Next, using Ohm’s law [29], the voltages on the scala vestibuli and scala tympani sides were calculated (Fig. 4A-C). The voltage difference reflects the pressure gradient between the scala vestibuli and scala tympani, which induces basilar membrane movement in the cochlear partition (Fig. 4D). Fig. 4A is a diagram of the cochlea in normal status; the gradient is 6V. Fig. 4B is a diagram of SSCD, and the additional impedance reflects the third window (R3rd) on the scala vestibuli side. The gradient is 8V (greater than the normal 6V), reflecting an increase in BC sound sensitivity. In a patient with otosclerosis, thus with a sclerotic area around the OW, ROW will rise, precluding AC current generation; thus, AC hearing falls. The AC must pass through the OW, which always serves as an impedance in AC. However, this is not the case for BC. A small proportion of BC energy (BC* in Fig. 4D) would normally enter through the OW but now follows other adjacent bony pathways that conduct better than the OW (Fig. 4E). Thus, it is more reasonable to configure otosclerosis as in Fig. 4C. The pressure gradient, 2 V, is smaller than the 6 V of Fig. 4A, and BC hearing thus decreases. However, if surgery mobilizes the OW, the configuration becomes that of Fig. 4A, and BC hearing improves.
The surgical outcomes were poor when otosclerosis involved the RW [22]. This is explained by our circuit model. If a lesion involves the RW, the RRW increases (Supplementary Fig. 3). In such a case, BC hearing is likely to be poor preoperatively (Supplementary Fig. 3A), and the prognosis would also be unfavorable. Even after successful surgery, the gradient (4V in Supplementary Fig. 3B) would be less than normal (6 V in Fig. 4A).
Overall, improved BC hearing after middle ear surgery does not reflect the recovery of a sensorineural component. Preoperative BC hearing may be underestimated for the reasons described above. In the clinic, if a patient presents with otosclerosis, and an otologist considers whether to perform surgery, the preoperative BC hearing at 1,000–2,000 Hz may be underestimated. For example, an apparent air-bone gap of 20 dB may in fact be about 30 dB. Such a difference affects decision-making. Therefore, considering the possibility of the improvement of BC hearing after surgery, the authors have recently chosen to offer stapes surgery to those otosclerosis patients.
BC hearing improvement after middle ear surgery is not rare, and BC hearing may be underestimated before surgery. This improvement is generally associated with larger postoperative AC hearing gains. Compared to ossiculoplasty patients, this improvement was more evident after stapes surgery, which may be associated with a renewed ability of the OW to function as a window. When considering whether to perform surgery, it should be borne in mind that the BC thresholds at 1,000–2,000 Hz may rise by about 10 dB.
▪ Bone conduction can improve when air conduction is enhanced after ear surgery.
▪ Improved bone conduction was most pronounced after stapes surgery.
▪ The improvement in bone conduction could be explained by a hypothetical circuit model and the third window theory.
▪ As the number of windows in the cochlea increases, bone conduction improves.
▪ In stapes surgery, bone conduction hearing at 1,000–2,000 Hz may rise by about 10 dB.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.21053/ceo.2022.01039.
Supplementary Table 1.
Correlation between the average AC gain (500–4,000 Hz) and BC gain at each frequency
Supplementary Fig. 1.
Pre- and postoperative audiograms. (A) Staged ossiculoplasty after canal wall up mastoidectomy (CWUM) cases. (B)
Staged ossiculoplasty cases after canal wall down mastoidectomy (CWDM) cases. (C) Ossiculoplasty without any prior middle ear surgery
cases. (D) Stapes surgery cases. The bars are the standard errors. AC, air conduction; BC, bone conduction.
Supplementary Fig. 2.
Audiograms of subjects exhibiting air conduction (AC) hearing gains ≤30 dB. (A) Staged ossiculoplasty after canal wall
up mastoidectomy (CWUM) cases. (B) Staged ossiculoplasty after canal wall down mastoidectomy (CWDM) cases. (C) Ossiculoplasty without
any prior middle ear surgery cases. (D) Stapes surgery cases. BC, bone conduction.
Supplementary Fig. 3.
In patients with severe otosclerosis, the round window (RW) is also sclerotic. (A) A sclerotic RW is associated with high
impedance; the pressure gradient is greatly reduced. (B) Even after stapes surgery, the RW impedance remains high. Note that the pressure
gradient is lower than that of Fig. 5A. Therefore, the surgical outcomes in patients with such severe otosclerosis may be somewhat unfavorable.
RSV, resistance of the scala vestibuli; RST, resistance of the scala tympani; RRW, resistance of the round window; ROW, resistance of the
oval window.
REFERENCES
1. Kileny PR, Zwolan TA, Slagar HK. Diagnostic audiology and electrophysiologic assessment of hearing. In : Flint PW, Francis HW, Haughey BH, Lesperance MM, Lund VJ, Robbins KT, editors. Cummings otolaryngology: head and neck surgery. 7th ed. Elsevier;2021. p. 2021–41.
2. Richter U, Brinkmann K. Threshold of hearing by bone conduction: a contribution to international standardization. Scand Audiol. 1981; 10(4):235–7.
3. Stenfelt S, Goode RL. Bone-conducted sound: physiological and clinical aspects. Otol Neurotol. 2005; Nov. 26(6):1245–61.

4. Khanna SM, Tonndorf J, Queller JE. Mechanical parameters of hearing by bone conduction. J Acoust Soc Am. 1976; Jul. 60(1):139–54.

5. Stenfelt S. Simultaneous cancellation of air and bone conduction tones at two frequencies: extension of the famous experiment by von Bekesy. Hear Res. 2007; Mar. 225(1-2):105–16.
6. Stenfelt S, Puria S, Hato N, Goode RL. Basilar membrane and osseous spiral lamina motion in human cadavers with air and bone conduction stimuli. Hear Res. 2003; Jul. 181(1-2):131–43.

7. Mauldin L, Jerger J. Auditory brain stem evoked responses to boneconducted signals. Arch Otolaryngol. 1979; Nov. 105(11):656–61.

8. Purcell D, Kunov H, Madsen P, Cleghorn W. Distortion product otoacoustic emissions stimulated through bone conduction. Ear Hear. 1998; Oct. 19(5):362–70.

9. Kobayashi T, Sakurai T, Okitsu T, Yuasa R, Kawase T, Kusakari J, et al. Labyrinthine fistulae caused by cholesteatoma: improved bone conduction by treatment. Am J Otol. 1989; Jan. 10(1):5–10.
10. Vijayendra H, Parikh B. Bone conduction improvement after surgery for conductive hearing loss. Indian J Otolaryngol Head Neck Surg. 2011; Jul. 63(3):201–4.

11. Kitahara T, Kamakura T, Ohta Y, Morihana T, Horii A, Uno A, et al. Chronic otitis media with cholesteatoma with canal fistula and bone conduction threshold after tympanoplasty with mastoidectomy. Otol Neurotol. 2014; Jul. 35(6):981–8.

12. Lee HS, Hong SD, Hong SH, Cho YS, Chung WH. Ossicular chain reconstruction improves bone conduction threshold in chronic otitis media. J Laryngol Otol. 2008; Apr. 122(4):351–6.

13. Milner RM, Weller CR, Brenman AK. Elevated bone conduction thresholds associated with middle ear fluid in adults. Int J Pediatr Otorhinolaryngol. 1983; Nov. 6(2):163–9.

14. Tuz M, Dogru H, Uygur K, Gedikli O. Improvement in bone conduction threshold after tympanoplasty. Otolaryngol Head Neck Surg. 2000; Dec. 123(6):775–8.

15. Kobayashi K, Kodama H, Takezawa H, Suzuki T, Kataura A. Elevation of bone conduction threshold in children with middle ear effusion. Int J Pediatr Otorhinolaryngol. 1988; Nov. 16(2):95–100.

16. Prodanovic S, Stenfelt S. Consequences of mastoidectomy on bone conducted sound based on simulations in a whole human head. Otol Neurotol. 2020; Oct. 41(9):e1158–66.

17. Stenfelt S. Acoustic and physiologic aspects of bone conduction hearing. Adv Otorhinolaryngol. 2011; 71:10–21.

18. Homma K, Du Y, Shimizu Y, Puria S. Ossicular resonance modes of the human middle ear for bone and air conduction. J Acoust Soc Am. 2009; Feb. 125(2):968–79.

20. Merchant SN, Rosowski JJ. Conductive hearing loss caused by thirdwindow lesions of the inner ear. Otol Neurotol. 2008; Apr. 29(3):282–9.

21. Ho ML, Moonis G, Halpin CF, Curtin HD. Spectrum of third window abnormalities: semicircular canal dehiscence and beyond. AJNR Am J Neuroradiol. 2017; Jan. 38(1):2–9.

22. Bae YJ, Shim YJ, Choi BS, Kim JH, Koo JW, Song JJ. “Third window” and “single window” effects impede surgical success: analysis of retrofenestral otosclerosis involving the internal auditory canal or round window. J Clin Med. 2019; Aug. 8(8):1182.

23. Rosowski JJ, Songer JE, Nakajima HH, Brinsko KM, Merchant SN. Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol. 2004; May. 25(3):323–32.

24. Lee SY, Bae YJ, Kim M, Song JJ, Choi BY, Koo JW. Changes in vestibulo-ocular reflex gain after surgical plugging of superior semicircular canal dehiscence. Front Neurol. 2020; Jul. 11:694.

25. Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope. 2005; Oct. 115(10):1717–27.

27. Quesnel AM, Ishai R, McKenna MJ. Otosclerosis: temporal bone pathology. Otolaryngol Clin North Am. 2018; Apr. 51(2):291–303.
28. Tonndorf J, Khanna S, Fingerhood BJ. The input impedance of the inner ear in cats. Ann Otol Rhinol Laryngol. 1966; Sep. 75(3):752–63.
29. Ohm GS. Die galvanische Kette, mathematisch bearbeitet. 1st ed. T.H. Riemann;1827.
Fig. 1.
Audiograms of subjects exhibiting air conduction hearing gains >30 dB. (A) Staged ossiculoplasty after canal wall up mastoidectomy (CWUM) cases. (B) Staged ossiculoplasty after canal wall down mastoidectomy (CWDM) cases. (C) Cases of ossiculoplasty without any prior middle ear surgery. (D) Stapes surgery cases. The bars are the standard errors. AC, air conduction; BC, bone conduction.
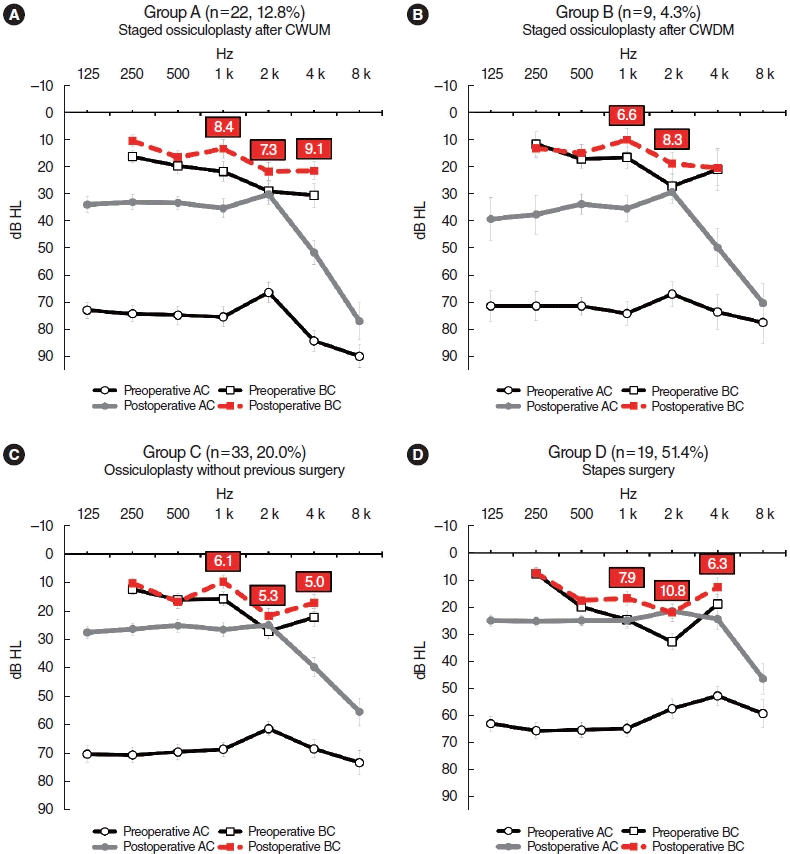
Fig. 2.
Correlations between the average air conduction (AC) hearing gains (500–4,000 Hz) and the average bone conduction (BC) hearing gains at each frequency. (A-C) In groups A to C, the highest correlations were apparent at 1,000 Hz. (D) In group D, the highest correlation was evident at 4,000 Hz. Group A underwent staged ossiculoplasty after canal wall up mastoidectomy (CWUM), group B underwent staged ossiculoplasty after canal wall down mastoidectomy (CWDM), group C underwent ossiculoplasty only (thus, they had no prior history of CWUM or CWDM), and group D received stapes surgery.
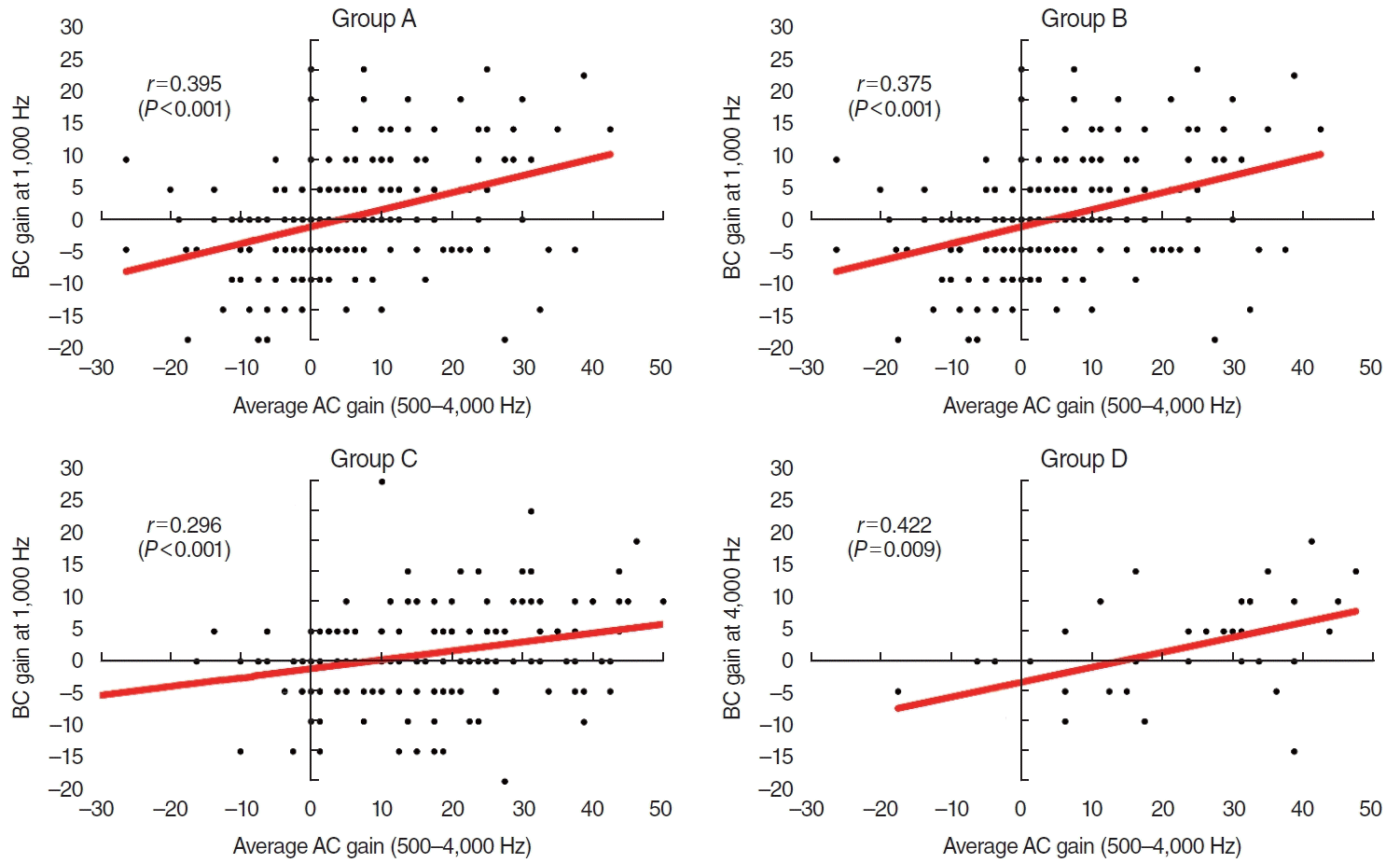
Fig. 3.
Audiograms of subjects in group D (stapes surgery group). (A) Eighteen subjects underwent stapes surgery to treat otosclerosis. (B) Nineteen underwent stapes surgery to treat other conditions such as congenital stapedial fixation. The bars are the standard errors. AC, air conduction; BC, bone conduction.
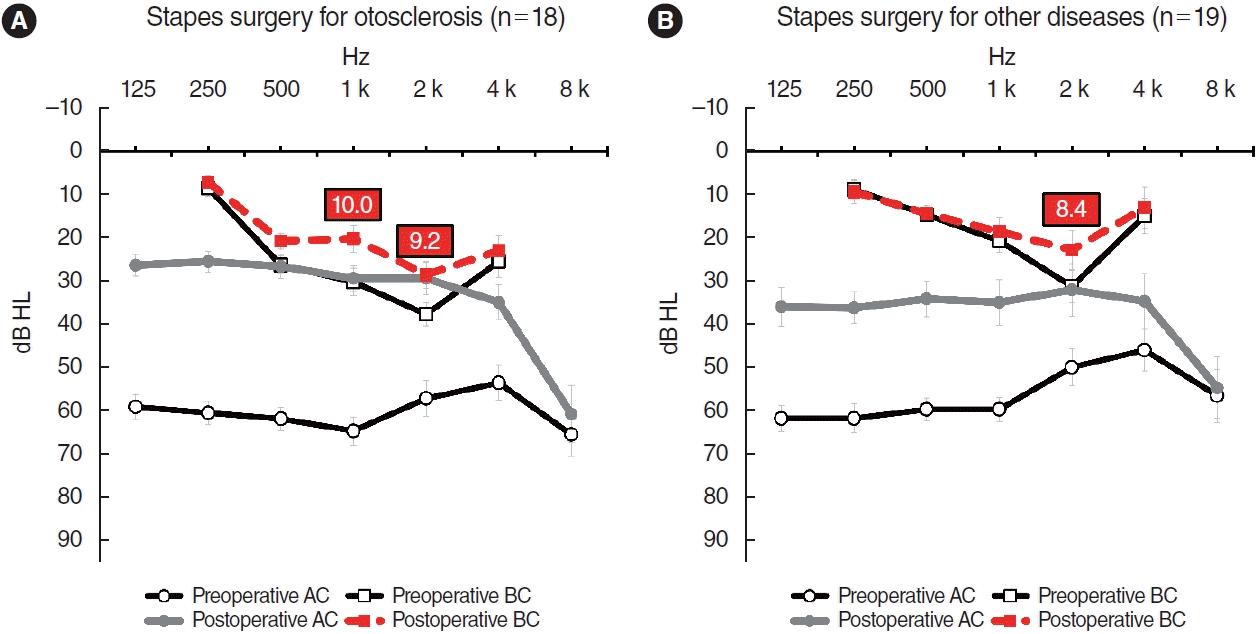
Fig. 4.
Hypothetical circuits showing the pressure differences between the scala vestibuli and scala tympani. The tops of the circuits represent the scala vestibuli and the bottoms the scala tympani. The values are arbitrary. (A) In the normal state, the gradient is 6V. (B) The model when superior semicircular canal dehiscence (SSCD) is in play. Another resistance (the third window) is added. The gradient is 8V; bone conduction (BC) hearing increases because of the larger pressure difference. (C) The model when otosclerosis is in play. The oval window (OW) has experienced a sclerotic change. Vibrational energy that normally passes through the OW now travels via other bony regions. In patients with otosclerosis, the OW does not serve as BC resistance. Schematics of cochlear air conduction (AC) and bone conduction (BC) (D, E). (D) The pressure gradient is created by the impedance difference between the scala vestibuli and scala tympani sides. BC* indicates conduction through the skull to the OW. (E) In patients with otosclerosis, BC* does not move through the OW but, rather, through other body regions, because the impedance of a sclerotic OW is very high. Conduction follows the path of least resistance. RSV, resistance of the scala vestibuli; ROW, resistance of the oval window; RST, resistance of the scala tympani; RRW, resistance of the round window; RW, round window.
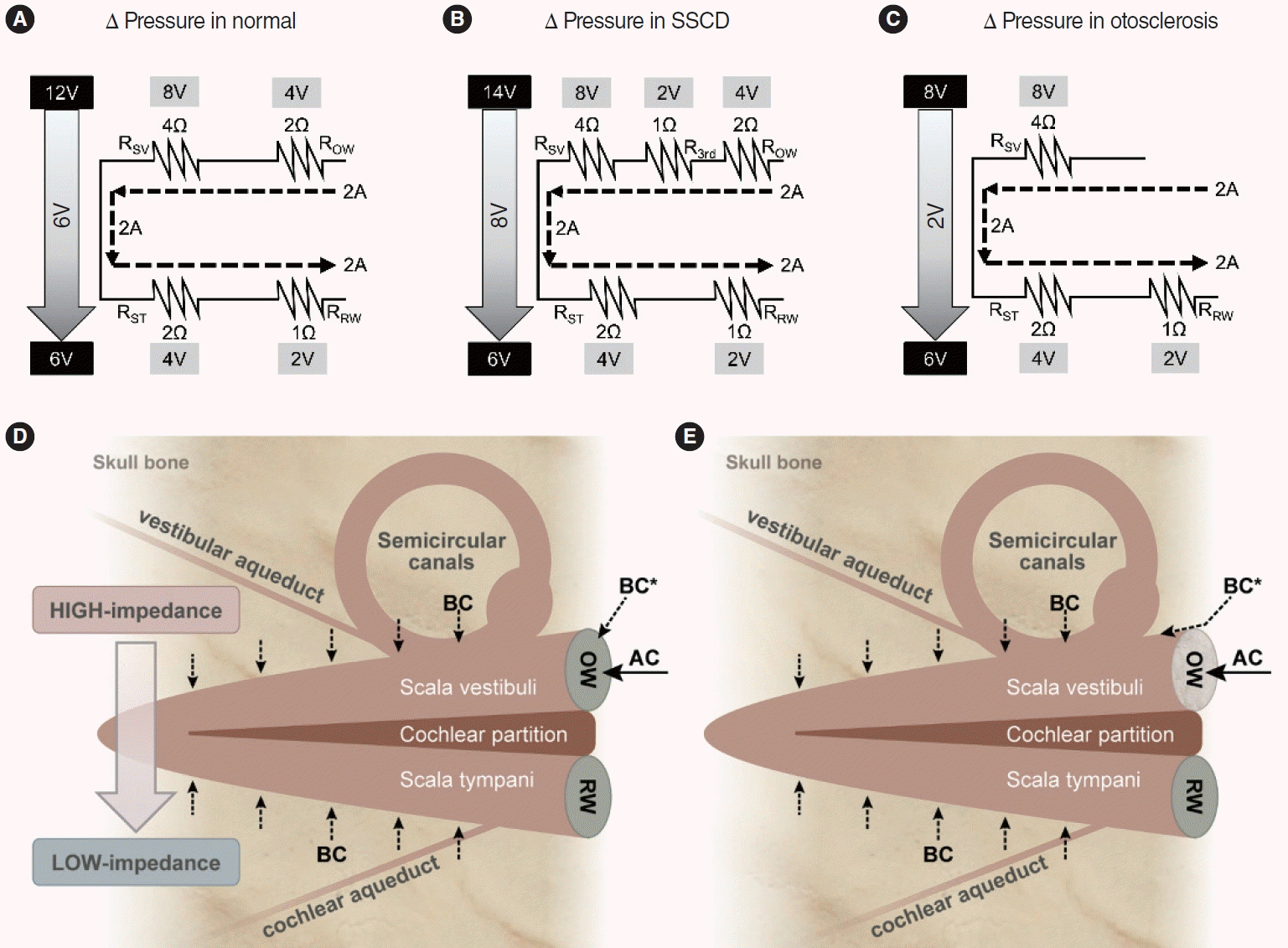
Table 1.
Demographic characteristics of the subjects
| Variable |
Ossiculoplasty |
Stapes surgery |
P-value | ||
|---|---|---|---|---|---|
| Group A (after CWUM) | Group B (after CWDM) | Group C | Group D | ||
| Sex | 0.227c) | ||||
| Male | 69 (40.1) | 97 (46.4) | 67 (40.6) | 11 (29.7) | |
| Female | 103 (59.9) | 112 (53.6) | 98 (59.4) | 26 (70.3) | |
| Age (yr) | 42.8±1.3 | 48.2±1.0 | 39.0±1.5 | 33.9±3.2 | <0.001d) |
| Side | 0.800c) | ||||
| Right | 90 (52.3) | 104 (49.8) | 89 (53.9) | 21 (56.8) | |
| Left | 82 (47.7) | 105 (50.2) | 76 (46.1) | 16 (43.2) | |
| AB gap (dB)a) | |||||
| Preoperative | 38.3±0.9 | 35.8±0.7 | 36.1±0.9 | 31.5±1.5 | 0.005d) |
| Postoperative | 29.5±1.0 | 30.2±0.8 | 20.4±0.9 | 12.0±1.8 | <0.001d) |
| BC elevationb) | |||||
| >10 dB | 50 (29.1) | 62 (29.7) | 46 (27.9) | 17 (45.9) | 0.178c) |
| >15 dB | 22 (12.8) | 19 (9.1) | 14 (8.5) | 10 (27.0) | 0.008c) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download