Abstract
Rapid adoption of a robotic approach as a minimally invasive surgery tool has enabled surgeons to perform more complex hepatobiliary surgeries than conventional laparoscopic surgery. Although various types of liver resections have been performed robotically, parenchymal transection is challenging as commonly used instruments (Cavitron Ultrasonic Surgical Aspirator [CUSA] and Harmonic) lack articulation. Further, CUSA also requires a patient-side assistant surgeon with hepatobiliary laparoscopic skills. We present a case report of total robotic right hepatectomy for multifocal hepatocellular carcinoma in a 70-year-old male using ‘Vessel Sealer’ for parenchymal transection. Total operative time was 520 minutes with a blood loss of ~400 mL. There was no technical difficulty or instrument failure encountered during surgery. The patient was discharged on postoperative day five without any significant complications such as bile leak. Thus, Vessel Sealer, a fully articulating instrument intended to seal vessels and tissues up to 7 mm, can be a promising tool for parenchymal transection in a robotic surgery.
In the era of minimally invasive surgery, more complex hepato-pancreatico-biliary (HPB) procedures are being performed robotically nowadays. Robotic surgeries have several advantages over conventional laparoscopic surgeries, such as three-dimensional vision with depth perception, magnified view, tremor filtration, and, more importantly, degrees of freedom of articulating instruments. The first robotic liver resection (minor) was reported in 2003 by Giulianotti et al. [1]. Since then, many centers have reported their experiences with robotic liver resections, including major liver resections (Couinaud’ segments four or more). However, in most of these cases, parenchymal transection was done using a Cavitron Ultrasonic Surgical Aspirator (CUSA) (Integra LifeSciences, Tullamore, Ireland), robotic compatible harmonic scalpel, or Maryland bipolar forceps (MBF) (Intuitive Surgical, Sunnyvale, CA, USA).
CUSA requires another experienced laparoscopic surgeon as an assistant, while a harmonic scalpel lacks articulation. MBF seem not optimally suited for bigger transection planes [2]. There is a paucity of literature on liver resection using an EndoWrist® One™ Vessel Sealer (Intuitive Surgical). Here we describe a case and the feasibility of liver parenchymal transection using a vessel sealer (VS) during total robotic right hepatectomy.
A 70-year-old non-alcoholic male presented with a history of pain in the right upper abdomen for the last four months, which was gradually progressive and relieved with medication to recure again. He had no history of jaundice, anorexia, weight loss, abdominal distension, or fever. His liver function tests were within normal limits.
A contrast-enhanced computed tomography (CECT) showed an ill-defined heterogeneous mass lesion of size ~4.8 cm × 3.9 cm × 4.0 cm having a non-rim arterial phase hyperenhancement with washout and enhancing capsule in delayed phase in segment VII of the liver. Another smaller lesion (1 cm) with similar enhancement patterns was noted in segment VIII on a background of the non-cirrhotic liver (Fig. 1). These lesions were further confirmed with a contrast-enhanced magnetic resonance imaging. No other lesions were found. His alpha-fetoprotein level was 3.93 ng/mL. Hepatitis B surface antigen and anti-hepatitis C antibody results were non-reactive. Hence, he was planned to undergo anatomical right hemi-hepatectomy robotically.
The patient was placed in a supine position with legs split. Pneumoperitoneum was created. Four robotic ports were placed in a straight line at the level of the umbilicus as follows: R1, right anterior axillary line; R2 (camera port) and R3 on either side of the umbilicus at a distance of ~7 cm; and R4 left anterior axillary line 7 cm from R3. Two assistant ports were used: A1, 12 mm infra-umbilical port and an A2, 5 mm port placed between R1 and R2 at the lower level forming a triangle (Fig. 2A–2C). A da Vinci Xi Surgical System (Intuitive Surgical) was docked from the right side. Instruments were placed as follows: MBF in arm R1, VS in arm R3, and Cadière forceps in R4.
After inspecting the peritoneal cavity for occult metastases, the falciform and right triangular ligament were divided and right hepatic vein (RHV) was identified. Calot’s dissection was done. The gallbladder was dissected out. Inflow control was taken using an intrafascial approach to loop the right portal vein (RPV) and the right hepatic artery (RHA) (Fig. 3, 4). Vascular clamps (bulldog) were applied. The ischemic line was marked and reconfirmed using intravenous indocyanine green dye (Fig. 5). Inferiorly, inferior vena cava (IVC) was identified. Short hepatic veins draining into the IVC were clipped and divided. At the hilar plate, RPV and RHA were clipped and divided (Fig. 6).
A rubber band technique was used to retract the liver. Parenchyma transection was done using a vessel sealer. MBF and VS were used for crushing the liver parenchyma. The VS was employed to seal all small vessels and glissonian pedicles. Tributaries of the middle hepatic vein from segments V and VIII were clipped and divided between Hem-o-lok clips (Teleflex Inc., Morrisville, NC, USA) (Fig. 7). After that, the right posterior and anterior sectoral ducts were divided using ICG cholangiography (firefly mode) (Fig. 8). Once all structures were divided at the hilum, further parenchymal transection was completed above the IVC in a similar manner. The specimen was left attached to RHV, which was then divided using endovascular staplers (Fig. 9).
The specimen was retrieved using a Pfannenstiel incision (Fig. 2D) and the robot was redocked. Ductal openings were closed with PDS 5-0. The hilar plate was also over-run. There was no apparent bile leak from the cut surface or hilum at the end of the procedure. Total operative time was 520 minutes with a console time of 400 minutes, including 130 minutes for parenchymal transection. Blood loss was ~400 mL without peri-operative transfusion requirement. The patient was discharged on postoperative day five without postoperative complications. Histopathology revealed two well-differentiated hepatocellular carcinomas, concurring with findings of CECT.
The utilization of robotic armamentarium is constantly expanding for HPB surgery. Advocates of robotic hepatobiliary surgery claim that depth perception, tremor filtration, improved dexterity, and wrist joint movement make it an inexpensive surgical instrument that can assist in the manipulation of complex bile ducts and blood vessels. The surgeon can sit comfortably on the console, allowing the tissue to be smoothly dissected and sutured accurately without feeling fatigued or stressed. An excellent visualization is the key to control intraoperative bleeding during mobilization and transection of the liver. Hence, a conversion to open surgery is less than a laparoscopic surgery [3].
Since the description of segmental liver resection in 2003 by Giulianotti et al. [1], the number and complexity of robotic liver resections have rapidly increased, especially in the last five years [4]. While earlier indications were limited to segmental, non-anatomical, and wedge resection, surgeons are now performing extended hemi-hepatectomies, hepatectomy for living donor liver transplantation, and segmental resection of posterosuperior segments with increasing experiences [5-8]. The International consensus statement has concluded that robotic hepatectomy is safe and feasible as a conventional open hepatectomy. It has less intra-operative blood loss, shorter hospital stay, and lower intra- and postoperative complications, although it has a longer operation time. Robotic hepatectomy also has effectiveness for liver malignancy lesions comparable to an open approach. There is no statistical difference in radical resection rate, overall survival, or recurrence rate regarding the oncological outcome between the two techniques [4]. The cost has always been the biggest challenge and limitation in robotic surgery. However, recent cost analysis studies of liver resection have suggested that higher intra-operative cost is balanced by shorter postoperative stay and lesser complications, thus reducing the overall cost [9-14].
Various techniques have been described for parenchymal transection during minimally invasive liver resections. The most common and preferred one is the CUSA, which provides precise transection of liver parenchyma, leaving only vascular and biliary structures to be clipped and divided. Apart from being costly, the drawback with CUSA in robotic liver surgery is that an assistant can only use it as it is not compatible with a da Vinci robotic surgical system. Hence, it requires an expert laparoscopic hepatobiliary surgeon to be present on the patient side. Other techniques include the use of a Harmonic scalpel and a robotic bipolar cautery. The problem with both Harmonic and CUSA is that they lack articulation as available in robotic instruments, while bipolar is not suitable for larger transection planes if not being simultaneously used with other energy devices.
An EndoWrist® One™ VS is a good alternative for parenchymal transection, although studies on its use are limited. It is a fully wristed robotic energy device optimal for the division of vessels up to 7 mm in diameter. Kingham et al. [15] have reported a few cases of robotic liver resection using VS. However, outcomes were not discussed for different transection techniques. Nota et al. [2] have used VS exclusively in their series of 70 liver resections. Among them, only 10 (14%) were major hepatectomies with a conversion rate of 7%. They reported that 14% suffered from a major complication [2]. They did not encounter any technical difficulty or instrument failure when using the VS. Thus, they have concluded that it is safe and feasible for parenchymal transection. In our experience, we have performed ten liver resections, including non-anatomical and minor resections using VS without bile leak or any other major complications. Besides liver surgery, VS has also been used in other abdominal surgeries. A retrospective analysis of 72 robotic procedures by Ortenzi et al. [16] has concluded that it is safe with decreased overall operative time. In a study by Kong et al. [17] of 17 patients of robotic gastrectomy for gastric cancer, it was concluded that the use of the VS in robotic gastrectomy was feasible, providing exemplary configuration in the direction of dissection, rapid learning curve, and comparable results between VS and conventional ultrasonic shear groups. At the same time, it can reduce inflammation with less albumin loss as possible benefits of the VS.
A VS can be proposed for dissection of tissue, bipolar coagulation, and transection of vessels and tissue bundles with a minimal lateral spread of thermal energy. Hence, there is minimal use of titanium clips during parenchymal transection. There is less dependency on an assistant surgeon for the same with a rapid learning curve. Besides, it can help dissect tissues in a few places without the need to replace the instrument, thus minimizing the time used in exchanging instruments in a robotic surgery.
The drawback of the proposed VS is that its jaws are broad and bulky. However, retraction of liver parenchyma with the help of a rubber band technique can help accommodate its jaws easily during transection and seal all pedicles. Furthermore, the modern version ‘vessel sealer extend’ or ‘Synchroseal’ has a slimmer jaw profile that will enable more delicate dissection like other robotic instruments.
In our case, we did not encounter any technical problems in using the VS. The patient was discharged with a smooth postoperative course without any complications such as bile leak. Thus, an EndoWrist® One™ VS is a safe and feasible method for parenchymal transection. However, more studies are needed to validate its role in major liver resections and living donor liver hepatectomies in the future.
REFERENCES
1. Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, et al. 2003; Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 138:777–784. DOI: 10.1001/archsurg.138.7.777. PMID: 12860761.
2. Nota CL, Molenaar IQ, te Riele WW, van Santvoort HC, Borel Rinkes IHM, Hagendoorn J. 2020; Parenchymal transection in robotic liver resection: results of 70 resections using the Vessel Sealer. Mini-invasive Surg. 4:74. DOI: 10.20517/2574-1225.2020.57.

3. Zhang L, Yuan Q, Xu Y, Wang W. 2020; Comparative clinical outcomes of robot-assisted liver resection versus laparoscopic liver resection: a meta-analysis. PLoS One. 15:e0240593. DOI: 10.1371/journal.pone.0240593. PMID: 33048989. PMCID: PMC7553328.

4. Liu R, Wakabayashi G, Kim HJ, Choi GH, Yiengpruksawan A, Fong Y, et al. 2019; International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol. 25:1432–1444. DOI: 10.3748/wjg.v25.i12.1432. PMID: 30948907. PMCID: PMC6441912.

5. Choi SB, Park JS, Kim JK, Hyung WJ, Kim KS, Yoon DS, et al. 2008; Early experiences of robotic-assisted laparoscopic liver resection. Yonsei Med J. 49:632–638. DOI: 10.3349/ymj.2008.49.4.632. PMID: 18729307. PMCID: PMC2615291.

6. Croner RS, Perrakis A, Brunner M, Matzel KE, Hohenberger W. 2015; Pioneering robotic liver surgery in Germany: first experiences with liver malignancies. Front Surg. 2:18. DOI: 10.3389/fsurg.2015.00018. PMID: 26052515. PMCID: PMC4440394.

7. Quijano Y, Vicente E, Ielpo B, Duran H, Diaz E, Fabra I, et al. 2017; Hepatobilio-pancreatic robotic surgery: initial experience from a single center institute. J Robot Surg. 11:355–365. DOI: 10.1007/s11701-016-0663-z. PMID: 28039607.

8. Giulianotti PC, Tzvetanov I, Jeon H, Bianco F, Spaggiari M, Oberholzer J, et al. 2012; Robot-assisted right lobe donor hepatectomy. Transpl Int. 25:e5–e9. DOI: 10.1111/j.1432-2277.2011.01373.x. PMID: 22029717.

9. Machado MAC, Lobo-Filho MM, Mattos BH, Ardengh AO, Makdissi FF. 2021; Robotic liver resection. Report of the first 50 cases. Arq Gastroenterol. 58:514–519. DOI: 10.1590/s0004-2803.202100000-92. PMID: 34909859.

10. Wong DJ, Wong MJ, Choi GH, Wu YM, Lai PB, Goh BKP. 2019; Systematic review and meta-analysis of robotic versus open hepatectomy. ANZ J Surg. 89:165–170. DOI: 10.1111/ans.14690. PMID: 29943881.

11. Ziogas IA, Giannis D, Esagian SM, Economopoulos KP, Tohme S, Geller DA. 2021; Laparoscopic versus robotic major hepatectomy: a systematic review and meta-analysis. Surg Endosc. 35:524–535. DOI: 10.1007/s00464-020-08008-2. PMID: 32989544.

12. Cortolillo N, Patel C, Parreco J, Kaza S, Castillo A. 2019; Nationwide outcomes and costs of laparoscopic and robotic vs. open hepatectomy. J Robot Surg. 13:557–565. DOI: 10.1007/s11701-018-0896-0. PMID: 30484059.

13. Daskalaki D, Gonzalez-Heredia R, Brown M, Bianco FM, Tzvetanov I, Davis M, et al. 2017; Financial impact of the robotic approach in liver surgery: a comparative study of clinical outcomes and costs between the robotic and open technique in a single institution. J Laparoendosc Adv Surg Tech A. 27:375–382. DOI: 10.1089/lap.2016.0576. PMID: 28186429. PMCID: PMC5397272.

14. Sham JG, Richards MK, Seo YD, Pillarisetty VG, Yeung RS, Park JO. 2016; Efficacy and cost of robotic hepatectomy: is the robot cost-prohibitive? J Robot Surg. 10:307–313. DOI: 10.1007/s11701-016-0598-4. PMID: 27153838.

15. Kingham TP, Leung U, Kuk D, Gönen M, D'Angelica MI, Allen PJ, et al. 2016; Robotic liver resection: a case-matched comparison. World J Surg. 40:1422–1428. DOI: 10.1007/s00268-016-3446-9. PMID: 26913732. PMCID: PMC4870111.

16. Ortenzi M, Ghiselli R, Baldarelli M, Cardinali L, Guerrieri M. 2018; Is the bipolar vessel sealer device an effective tool in robotic surgery? A retrospective analysis of our experience and a meta-analysis of the literature about different robotic procedures by investigating operative data and post-operative course. Minim Invasive Ther Allied Technol. 27:113–118. DOI: 10.1080/13645706.2017.1329212. PMID: 28604140.

17. Kong SH, Kim TH, Huh YJ, Oh SY, Ahn HS, Park SY, et al. 2017; A feasibility study and technical tips for the use of an articulating bipolar vessel sealer in da Vinci robot-assisted gastrectomy. J Laparoendosc Adv Surg Tech A. 27:1172–1179. DOI: 10.1089/lap.2017.0093. PMID: 28622078.

Fig. 1
Computerised Tomographic images of hepatocellular carcinoma. (A, B) Lesion in segment VII in arterial and portal venous phase. (C, D) Another small lesion in segment VIII which is better appreciated in portal venous phase (circles).
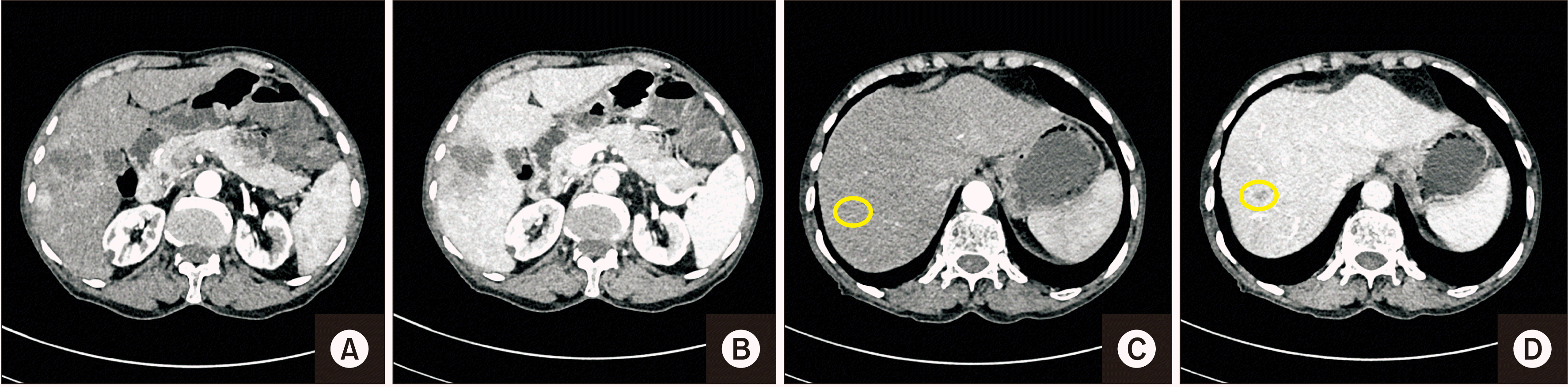
Fig. 2
(A) Port position: diagram: R1– R4, Robotic ports; A1, A2, Assistant ports. (B, C) Intraoperative image of port placement. (D) Intraoperative image showing port sites with Pfannenstiel scar at the end of the procedure.

Fig. 3
(A–D) Sequential depiction of dissection and looping of the right portal vein with blue vascular sling (D). Small caudate vein is clipped and divided (arrow).
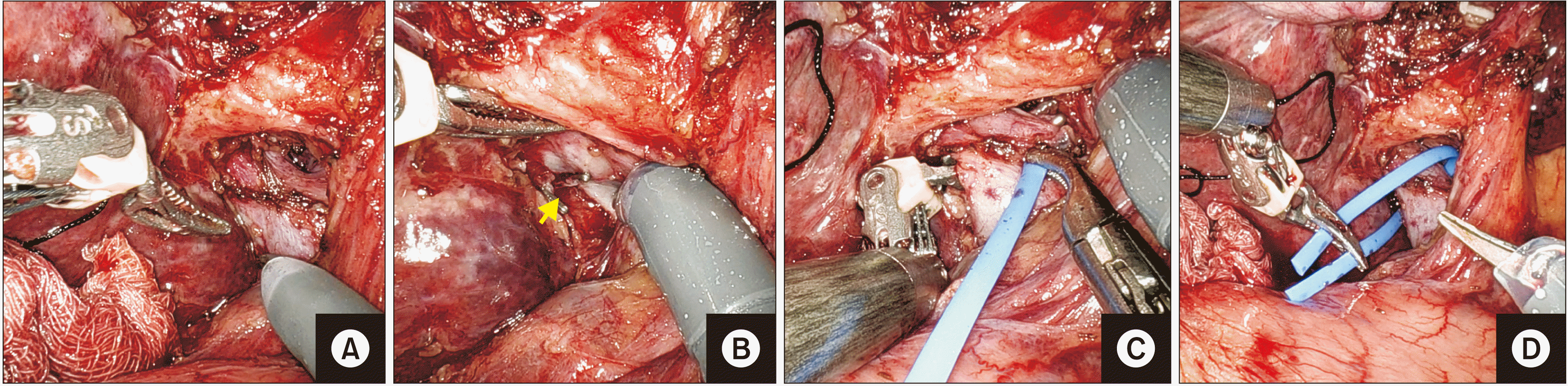
Fig. 4
(A–D) Sequential depiction of dissection and looping of the right hepatic artery (arrow) with red vascular sling (D).
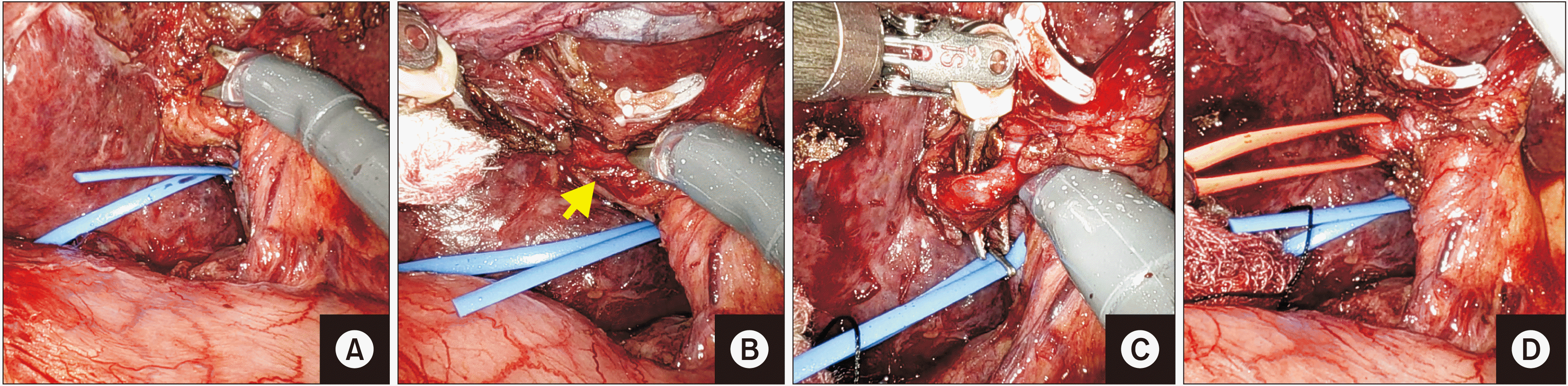
Fig. 5
(A, B) Application of vascular clamps on right portal vein and hepatic artery to occlude the inflow to the right liver. (C, D)
Ischemic line being marked. (E, F) Confirming the ischemic line using intravenous indocyanine green dye in firefly mode.
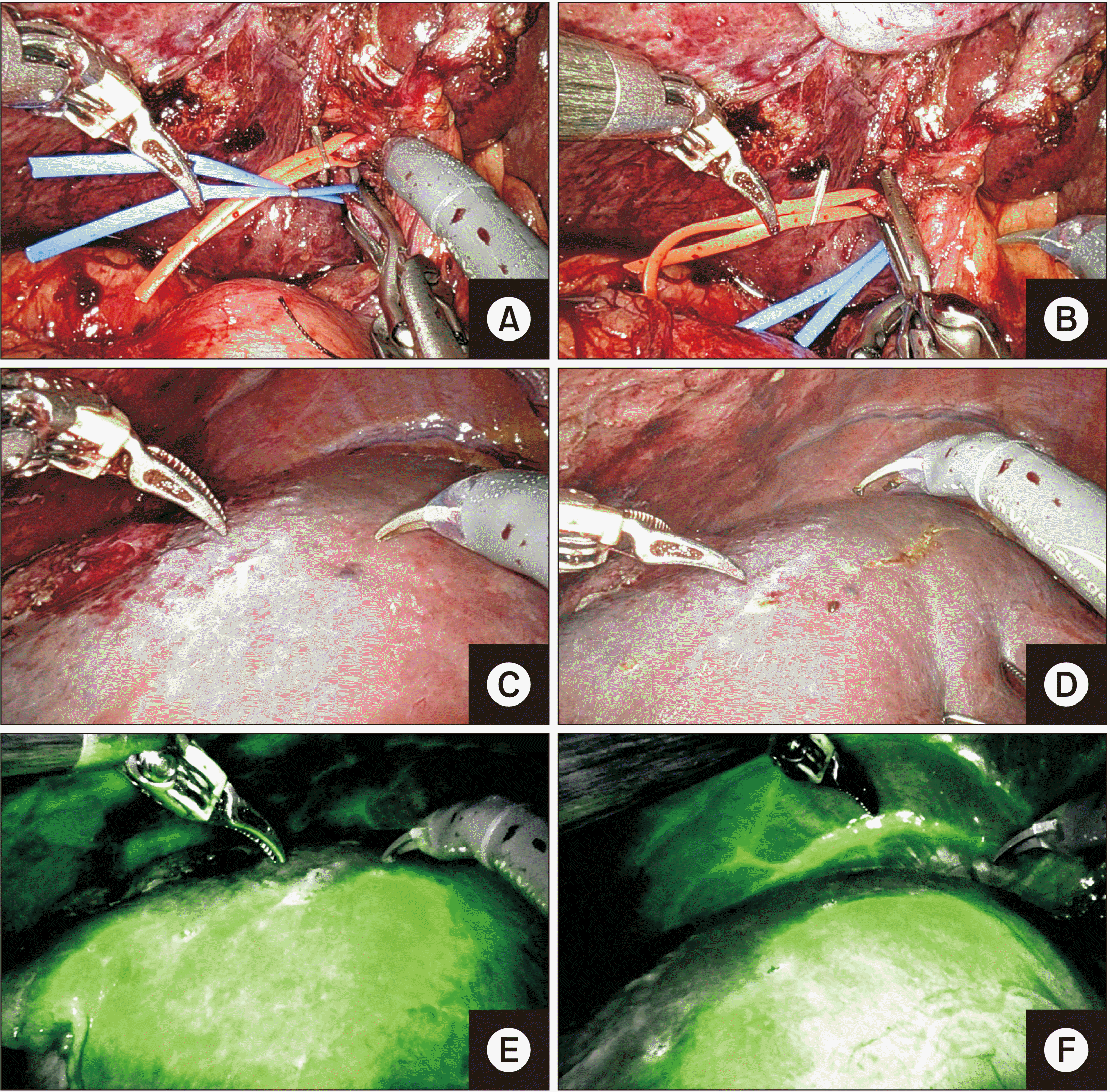
Fig. 6
(A, B) Clipping and dividing the right hepatic artery (arrow). (C, D) Clipping and dividing the right portal vein (arrow).
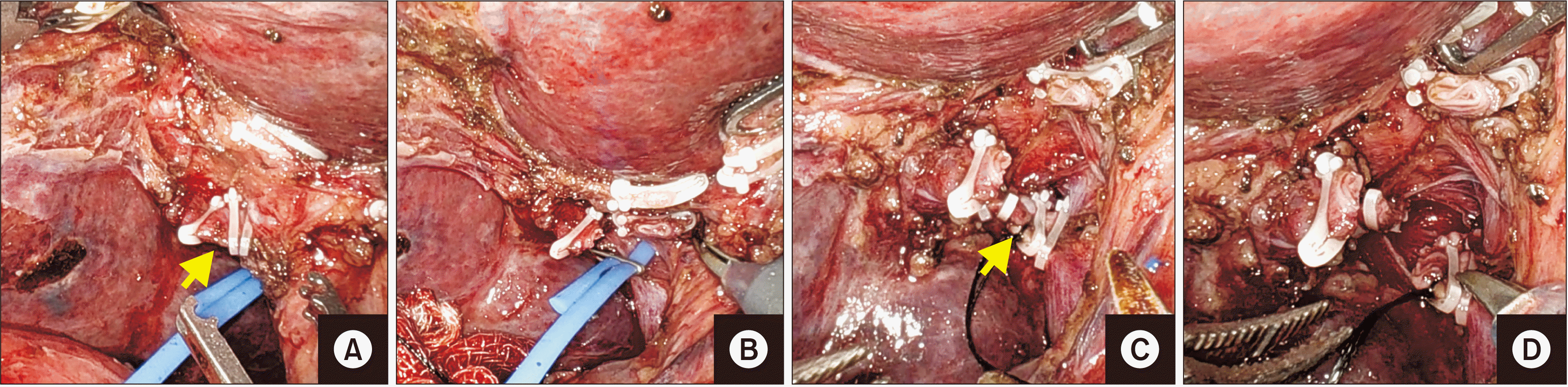
Fig. 7
(A–C) Sequential depiction of clipping and division of segment V vein (V5, arrow). (D–F) Sequential depiction of clipping and division of segments VIII vein (V8, arrow).

Fig. 8
Dissection at hilar plate and division of right hepatic duct. (A) Hilar plate after parenchymal transection. (B) Firefly mode to confirm right anterior sectoral duct (RASD) and right posterior sectoral duct (RPSD). (C) RASD being dissected with Maryland forceps and clipped. (D) RASD clipped and divided (arrow). (E) RPSD after being divided sharply with scissors (arrow). (F) Firefly mode to confirm leak of indocyanine green dye from the RPSD (which was later closed with PDS 5-0).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download