Abstract
Backgrounds/Aims
Central pancreatectomy (CP) is associated with a higher rate of postoperative pancreatic fistula (POPF), and it is less preferred over distal pancreatectomy (DP). We compared the short- and long-term outcomes between CP and DP for low-grade pancreatic neck and body tumors.
Methods
This was a propensity score-matched case-control study of patients who underwent either CP or DP for low-grade pancreatic neck and body tumors from 2003 to 2020 in a tertiary care unit in southern India. Patients with a tumor >10 cm or a distal residual stump length of <4 cm were excluded. Demographics, clinical profile, intraoperative and postoperative parameters, and the long-term postoperative outcomes for exocrine and endocrine insufficiency, weight gain, and the 36-Item Short Form Survey (SF-36) quality of life questionnaire were compared.
Results
Eighty-eight patients (CP: n=37 [cases], DP: n=51 [control]) were included in the unmatched group after excluding 21 patients (meeting exclusion criteria). After matching, both groups had 37 patients. The clinical and demographic profiles were comparable between the two groups. Blood loss and POPF rates were significantly higher in the CP group. However, Clavien-Dindo grades of complications were similar between the two groups (p = 0.27). At a median follow-up of 38 months (range = 187 months), exocrine sufficiency was similar between the two groups. Endocrine sufficiency, weight gain, SF-36 pain control score, and general health score were significantly better in the CP group.
The type of pancreatic resection is based on the location of the tumor. Tumor of the head and uncinate process of the pancreas is managed with pancreaticoduodenectomy (PD), and tumor of the tail of the pancreas is managed with distal pancreatectomy (DP) with or without splenectomy. However, tumor of the pancreatic neck and body can be managed with viable alternative procedures, such as central pancreatectomy (CP) without oncological compromise [1]. With the increasing use of cross-sectional imaging, an increasing number of low-grade tumors are being diagnosed and managed [2]. The indolent nature of these low-grade pancreatic tumors and the low recurrence rate make CP an effective alternative to distal pancreatic resection [3]. The disadvantage of DP for low-grade pancreatic tumors is that the normally functioning pancreatic parenchyma is sacrificed with no added oncologic benefit. Dagradi and Serio performed the first central pancreatic resection and reconstruction in 1982 [4]. Since then, many surgeons have reported their experiences with CP [4]. The rate of postoperative pancreatic fistula (POPF) after CP is high because there are two cut surfaces and a small-diameter pancreatic duct in the distal pancreatic stump that need to be managed after CP [5,6]. Some reports have compared the outcomes of CP with those of DP [6]; hence, we wanted to compare the short- and long-term outcomes of CP with those of DP.
A retrospective, case-control, propensity score-matched study was conducted using a prospectively maintained database from January 2003 to December 2020 at a tertiary care center in India. The study included patients who underwent either CP (case group) or DP (control group) for low-grade tumors of the pancreatic neck or proximal body. Patients who underwent PD, those with tumor size >10 cm in its largest dimension, remaining distal pancreatic stump length <4 cm, and patients who underwent multivisceral resection were excluded. The demographic, clinical, intraoperative, and immediate postoperative outcomes were reviewed.
Postoperative complications, such as POPF, delayed gastric emptying (DGE), and postpancreatectomy hemorrhage (PPH), were recorded according to the classification of the International Study Group of Pancreatic Surgery (ISGPS). Morbidities were recorded according to the Clavien-Dindo classification. Long-term outcomes, including weight gain, endocrine or exocrine insufficiency, and long-term quality of life using the 36-Item Short Form Survey (SF-36), were assessed. Exocrine and endocrine insufficiency was recorded as absent, unchanged, worsening, or new-onset.
All patients were evaluated with either contrast-enhanced computed tomography or magnetic resonance imaging. Endoscopic ultrasound became available later during the study period and was used in patients in whom there was a dilemma about the diagnosis and management. All patients with preoperative suspicion of pancreatic neuroendocrine tumor were evaluated with DOTAnoc-PET imaging and serum chromogranin levels.
The initial surgical steps were the same in both groups. All patients were operated via a midline incision. The lesser sac was opened, preserving the gastro-epiploic arcade. The tumor was evaluated for resectability. The duodenum was kocherized, and a tunnel was created behind the pancreatic neck on the anterior surface of the portal vein. The posterior surface of the pancreas was separated from the splenic vein to a point of transaction, approximately 10 mm from the tumor.
After performing the initial steps mentioned above, the splenic artery was looped and ligated at its origin. The splenic vein was looped and divided at the site of pancreatic transection. The pancreas was divided by electrocautery, and the proximal end was closed with 2-0 non-absorbable horizontal mattress sutures. The distal pancreatic stump was dissected via the antegrade approach along with the posterior bed. The spleen was resected en bloc.
After performing similar initial steps mentioned above, we proceeded with the dissection toward the tail of the pancreas, safeguarding the splenic artery and vein. When the tumor was sufficiently dissected from the splenic vessels nearly 10 mm distal to the distal extent of the tumor, the distal end was also transected while maintaining a 10 mm margin. The entire specimen was then sent for frozen section evaluation of resection margin status and gross identification of the tumor type. After histological confirmation of the negative margin status, we closed the proximal end in two layers and performed pancreaticojejunostomy for the distal end in most cases. When the pancreatic duct was visible, we preferred performing duct-to-mucosa anastomosis for pancreaticojejunostomy; and when the pancreatic duct was very small and could not be identified separately, we performed dunking anastomosis. We performed trans-jejunal external stenting in all cases where we could identify the duct with a 5-Fr infant feeding tube. Postoperatively, a somatostatin analog (octreotide) was used at the surgeon’s discretion.
During follow-up, patients were evaluated by performing blood glucose and HBA1c monitoring. Fecal elastase measurement was not routinely performed.
Postoperative morbidity (POPF, DGE, and PPH), mortality, and hospital stay were the primary outcomes. Weight gain, exocrine insufficiency, endocrine insufficiency, and quality of life (SF-36) were the secondary outcomes. Endocrine insufficiency was defined as diabetes mellitus requiring medical treatment and/or serum fasting glucose levels of more than 126 mg/dL. Exocrine insufficiency was defined as presence of fatty, frothy stools, weight loss and/or requirement of pancreatic enzyme supplements [7]. The secondary outcomes (endocrine and exocrine insufficiency) were assessed at a cross-sectional time point in September 2021 and compared with the preoperative levels.
Data were analyzed with IBM SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Cases were matched to controls using propensity score matching (1 : 1, the nearest neighbor method) with respect to patient age, sex, and American Society of Anesthesiologists (ASA) grade. Student’s t-test or Mann–Whitney U test was used for continuous variables, and the chi-square test or Fisher’s exact test was used for categorical variables. A two-tailed p-value less than 0.05 was considered significant.
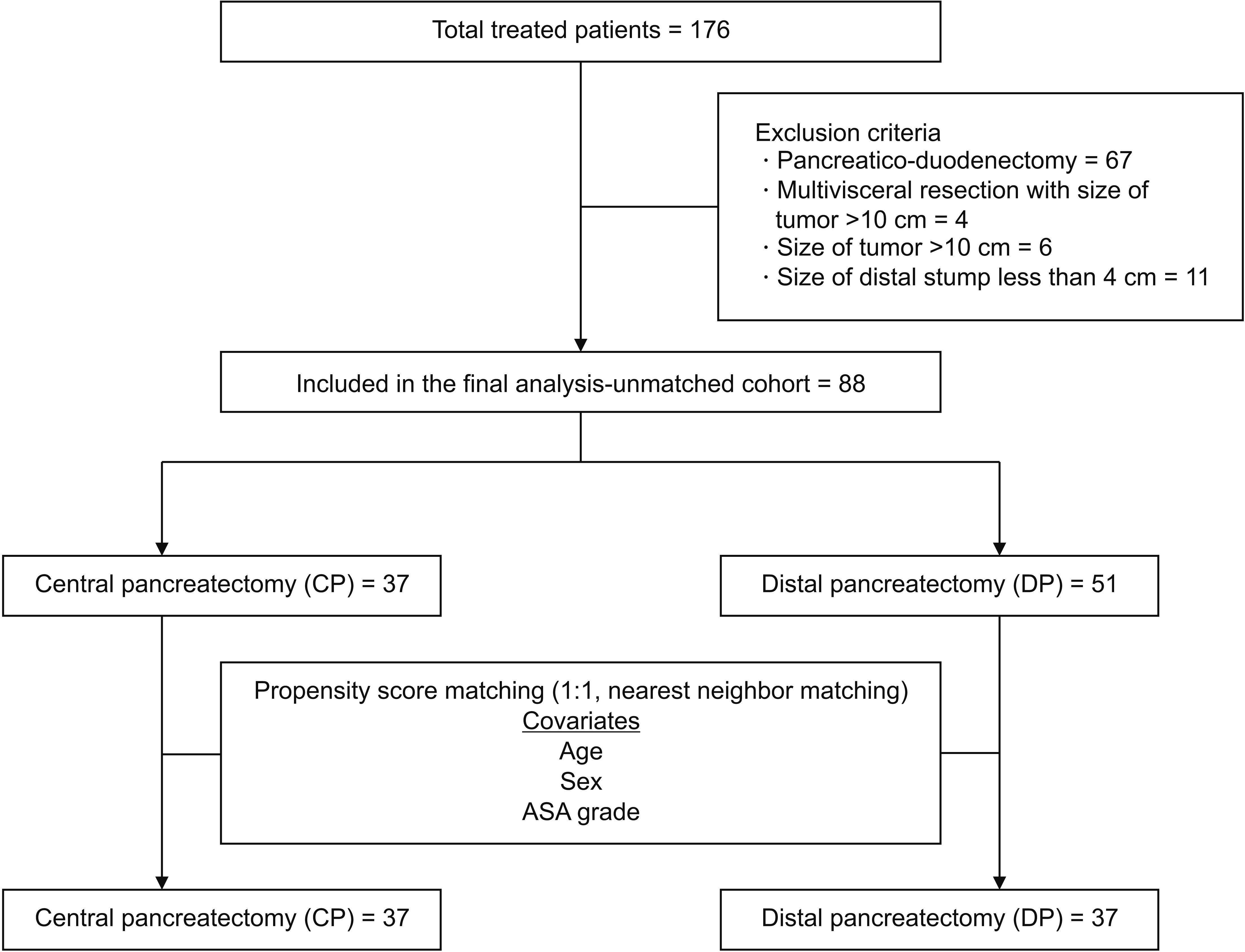
In 18 years (from 2003 to 2020), a total of 176 patients underwent surgery for low-grade pancreatic tumors. Of these, 67 patients underwent PD, and the remaining 109 patients underwent either CP (n = 37) or DP (n = 72). Eighty-eight patients (cases = 37, controls = 51) formed the unmatched study cohort after 21 patients were excluded based on the exclusion criteria. After propensity score matching, 74 patients (cases n = 37, controls n = 37) formed the matched cohort (Fig. 1).
Baseline clinical demographic parameters, such as age, sex, and preoperative comorbidities, such as diabetes, hypertension, ASA grade, and preoperative symptoms, were comparable between the two groups in both matched and unmatched study cohorts (Table 1). The duration of surgery, pancreatic duct diameter, and pancreatic texture were similar between the two groups in both matched and unmatched study cohorts.
The size of the lesion was significantly smaller in the case group in both matched and unmatched study cohorts (4 cm vs. 7 cm; p < 0.001), and the length of the residual pancreatic stump was significantly longer in the case group (5 cm vs. 4 cm; p < 0.001). It was found that blood loss was not significantly different in the unmatched study cohort, but it was significantly greater in the case group in the matched study cohort (Table 2).
Compared to the unmatched study cohort, the matched study cohort had a significantly higher rate of POPF in the case group. However, the clinically relevant POPF rates (ISGPS grades B & C) were similar between the two groups in both matched and unmatched study cohorts. Similarly, surgical site infection (SSI), DGE, and postoperative bleeding rates were similar between the two groups in both matched and unmatched study cohorts (Table 3).
Compared to the control group, the case group had significantly lower endocrine insufficiency and significantly better weight gain in both matched and unmatched study cohorts. Among the factors in SF-36, pain and general health parameters were significantly better in the case group than in the control group in both matched and unmatched study cohorts (Table 4).
All other parameters, such as exocrine insufficiency and other SF-36 parameters, were comparable between the two groups in matched and unmatched study cohorts.
The conventional approach for a neck or body lesion of the pancreas is either DP or PD [8]. This approach results in loss of normal functioning parenchyma, which can lead to insufficiency of pancreatic function with no added oncological benefit. Recent novel approaches for pancreatic parenchyma preservation, i.e., pancreatic enucleation, duodenum-sparing resection of the head of the pancreas, and CP, have become part of the surgeon’s arsenal, particularly for low-grade tumors [9].
A prerequisite for considering CP is that the benign nature of the lesion must be confirmed either preoperatively or intraoperatively. The presence of an invasive carcinoma during the final histopathological examination may necessitate reoperation [8]. A frozen section histological examination of the resected specimen was performed in all patients who underwent CP to rule out malignancy as well as negative margin status in the present study. Gao et al. [10] reported about CP in nine cases of ductal adenocarcinoma of the pancreas.
For large tumors and pancreatic tail tumors, DP may be the only surgical option available. We categorically excluded such patients from the present study to reduce selection bias. In a review of the literature, none of the other studies have specified such strict exclusion criteria.
In the last decade, more studies have reported about CP by minimally invasive surgery (laparoscopic or robotic) with acceptable morbidity and mortality [11,12]. However, all patients in the present study underwent open-technique CP.
After resection, one of the critical issues in CP is the management of two pancreatic stumps. The proximal stump can be managed by suture ligation, or division with a stapler or pancreatico-enteric anastomosis, falciform or omental patch reinforced suture closure [5]. The challenging aspect of CP is the management of the distal pancreatic stump, given the short distal stump length and the small size of the pancreatic duct. Usually, the distal pancreatic stump is managed by pancreatico-enteric anastomosis either by pancreaticojejunostomy (PJ) or pancreaticogastrostomy (PG) [13]. As a standard recommendation, stump less than 5 cm is a contraindication for CP. However, at our institute, we extended our criteria to perform CP even in patients with a 4-cm distal pancreatic stump length.
The reported POPF rates after PG and PJ after CP range from 0% to 36% and 0% to 63%, respectively [14]. A meta-analysis of studies on PD showed that PG was not superior to PJ with respect to POPF. However, in multicentre randomized controlled trials, the incidence of POPF was lower in patients undergoing PG than in patients undergoing PJ [15]. However, comparative studies between PG and PJ after CP have not been performed. In the present study, duct-to-mucosa PJ was preferred to dunking PJ when PD could be identified separately. Interestingly, Wayne et al. reported CP without anastomosis in 10 patients without POPF [14]. However, the length and status of the distal stump were not reported. Sauvanet et al. [16] also reported that the distal pancreatic stump was oversewn without an enteric anastomosis when the stump was small or atrophic. Recently, reports of end-to-end anastomosis of proximal and distal pancreatic stumps have been presented [17].
In the present study, the overall morbidity (Clavien-Dindo classification) or clinically significant morbidity (Clavien-Dindo grade 3 or greater) was similar between the two groups (unmatched study cohort 10.8% vs. 5.9%, p = 0.182; matched study cohort 10.8% vs. 5.4%, p = 0.158). In the CP group, two patients underwent pigtail catheter drainage of collection (Clavien-Dindo grade 3a) following POPF and one patient had multiple organ dysfunction (grade 4b). In the unmatched DP group, two patients (grade 3a) underwent pigtail catheter drainage and one patient developed single organ dysfunction (grade 4a). However, in the matched group, only two patients had grade 3a complication. The overall POPF rate was significantly higher in the CP group (unmatched study cohort 43.2% vs. 25.5%, p = 0.08; matched study cohort: 43.2% vs. 21.6%, p = 0.047). However, the rate of clinically relevant POPF was similar between the groups. However, a meta-analysis by Regmi et al. [18] (relative risk 1.64, p < 0.001) and Dragomir et al. [19] (odds ratio 2.24, p < 0.0001) showed a significantly higher incidence of clinically relevant fistulas after CP [17]. This observed difference might be due to our institutional practice of trans-jejunal external stenting of PD.
Other complications, such as PPH (unmatched study cohort 2.7% vs 0%, p = 0.238; matched study cohort 2.7% vs. 0%, p = 0.314), were comparable between the two groups in contrast to the previously reported series with a significantly higher risk of postoperative bleeding after CP [18]. Postoperative hospital stay was also similar between the two groups. These results could be due to lower rates of clinically relevant POPF after CP in our study. DGE, SSI, and pulmonary complications were comparable between the two groups. In the CP group, there was one case of postoperative mortality related to POPF.
Long-term outcomes, such as endocrine insufficiency, were significantly lower in the CP group, similar to the reported literature. However, exocrine insufficiency was similar between the two groups. The reported meta-analyses showed that CP was associated with significantly less exocrine insufficiency [18,19]. The incidence of postoperative exocrine insufficiency after CP varies widely, as it depends on the pre-existing pancreatic abnormality, the extent of the resection, and the presence of chronic pancreatitis [18]; and it also depends on the type of reconstruction (more in PG compared to PJ). In the CP group of our study, long-term outcomes, such as endocrine insufficiency (p = 0.532), exocrine insufficiency (p = 0.653), and weight gain (p = 0.395), after dunking and duct-to-mucosa anastomoses were similar between the two groups. As the sample size was small in the groups (dunking anastomosis, n = 18; duct-to-mucosa anastomosis, n = 19), it was difficult to determine which anastomosis could provide better long-term outcomes after CP.
Limited literature is available on the long-term quality of life after CP [4,19] The quality of life was similar between the CP and DP groups based on EORTC- quality of life [20]. The present study also compared the quality of life (SF-36 questionnaire) between the CP and DP groups. The general health and pain components of SF-36 were significantly better in the CP group. This could be explained by the statistically significant weight gain in the CP group at the long-term follow-up.
Our study is one of the largest series of CP resections in the subcontinent. However, the study has a few limitations. First, the retrospective design of the study and a large time span for sample acquisition may have introduced some confounders, leading to selection bias. Therefore, we used propensity score matching to reduce potential selection bias and confounding bias. Second, the size of the lesions could be one of the important factors for determining the surgical plan. The resulting sample size after including lesion size as one of the criteria for propensity score matching was small to compare the groups. Therefore, the size of the lesions was not included in propensity score matching. Third, no quantitative assessment criteria were used to diagnose exocrine insufficiency, which could have resulted in reporting bias. The major strength of the study is that, despite the small sample size, we included only those patients in the analysis who had been offered either CP or DP based on the surgeon’s discretion.
None of the patients in the present study had a recurrence during a median follow-up of 22 months (range, 11–118 months).
In conclusions, CP has a high morbidity rate, but the clinically significant morbidity and mortality rates are apparently similar when compared to those of DP. However, this needs to be validated by performing a larger sample study. CP has better endocrine sufficiency and similar exocrine sufficiency compared to DP. It is an oncologically non-inferior alternative procedure to DP with a good long-term quality of life, especially for low-grade pancreatic tumors.
ACKNOWLEDGEMENTS
We thank Prof. Vinay Kumar Kapoor for peer reviewing this manuscript and insightful discussions and valuable suggestions.
REFERENCES
1. Lee DH, Jang JY. 2020; ASO author reflections: central pancreatectomy versus distal pancreatectomy and pancreaticoduodenectomy for benign or low-grade malignant neoplasms: a propensity score-matched study with long-term functional outcomes and pancreas volumetry. Ann Surg Oncol. 27:1225–1226. DOI: 10.1245/s10434-019-08133-w. PMID: 31965371.

2. Iacono C, Verlato G, Ruzzenente A, Campagnaro T, Bacchelli C, Valdegamberi A, et al. 2013; Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 100:873–885. DOI: 10.1002/bjs.9136. PMID: 23640664.

3. Mahseeri M, Alqaiseieh A, Alkhader D, Halbony H, Albreazat M, Abualhaj S. 2021; Central pancreatectomy for solid pseudopapillary neoplasm: a pancreatic-preserving procedure. Int J Surg Case Rep. 79:91–93. DOI: 10.1016/j.ijscr.2021.01.009. PMID: 33444966. PMCID: PMC7806954.

4. Lv A, Qian HG, Qiu H, Wu JH, Hao CY. 2018; Is central pancreatectomy truly recommendable? A 9-year single-center experience. Dig Surg. 35:532–538. DOI: 10.1159/000485806. PMID: 29275422.

5. Girish S, Kapil N, Kannan N. 2021; Central pancreatectomy: significance of vascularity on anastomotic integrity and a note on reconstructive technique. Cureus. 13:e18617. DOI: 10.7759/cureus.18617.

6. Lee DH, Han Y, Byun Y, Kim H, Kwon W, Jang JY. 2020; Central pancreatectomy versus distal pancreatectomy and pancreaticoduodenectomy for benign and low-grade malignant neoplasms: a retrospective and propensity score-matched study with long-term functional outcomes and pancreas volumetry. Ann Surg Oncol. 27:1215–1224. DOI: 10.1245/s10434-019-08095-z. PMID: 31898101.

7. Nag HH, Nekarakanti PK, Arvinda PS, Sharma A. 2022; Laparoscopic versus open surgical management of patients with chronic pancreatitis: a matched case-control study. J Minim Access Surg. 18:191–196. DOI: 10.4103/jmas.JMAS_183_20. PMID: 33885009. PMCID: PMC8973484.

8. Johnson MA, Rajendran S, Balachandar TG, Kannan DG, Jeswanth S, Ravichandran P, et al. 2006; Central pancreatectomy for benign pancreatic pathology/trauma: is it a reasonable pancreas-preserving conservative surgical strategy alternative to standard major pancreatic resection? ANZ J Surg. 76:987–995. DOI: 10.1111/j.1445-2197.2006.03916.x. PMID: 17054548.

9. Brown KM, Shoup M, Abodeely A, Hodul P, Brems JJ, Aranha GV. 2006; Central pancreatectomy for benign pancreatic lesions. HPB (Oxford). 8:142–147. DOI: 10.1080/13651820510037611. PMID: 18333263. PMCID: PMC2131409.

10. Gao H, Liu T, Wang G, Gao Y, Yin L, Peng Y, et al. 2019; Central pancreatectomy for early-stage pancreatic ductal adenocarcinoma: a single-center case-control study. Langenbecks Arch Surg. 404:175–182. DOI: 10.1007/s00423-019-01766-1. PMID: 30826926.

11. Dokmak S, Aussilhou B, Paci M, Ftériche FS, Cros J, Maire F, et al. 2019; Extended laparoscopic central pancreatectomy with clamping of the mesentericoportal vein and resection of the splenic vessels for a large solid pseudopapillary tumor. Ann Surg Oncol. 26:3709–3710. DOI: 10.1245/s10434-019-07689-x. PMID: 31407182.

12. Senthilnathan P, Gul SI, Gurumurthy SS, Palanivelu PR, Parthasarathi R, Palanisamy NV, et al. 2015; Laparoscopic central pancreatectomy: our technique and long-term results in 14 patients. J Minim Access Surg. 11:167–171. DOI: 10.4103/0972-9941.158967. PMID: 26195873. PMCID: PMC4499920.

13. Kang P, Wang Z, Leng K, Zhong X, Wang H, Wan M, et al. 2017; Binding pancreaticogastrostomy anastomosis in central pancreatectomy: a single center experience. Medicine (Baltimore). 96:e8354. DOI: 10.1097/MD.0000000000008354. PMID: 29137016. PMCID: PMC5690709.
14. Wayne M, Neragi-Miandoab S, Kasmin F, Brown W, Pahuja A, Cooperman AM. 2009; Central pancreatectomy without anastomosis. World J Surg Oncol. 7:67. DOI: 10.1186/1477-7819-7-67. PMID: 19719851. PMCID: PMC2743692.

15. Lyu Y, Li T, Cheng Y, Wang B, Chen L, Zhao S. 2018; Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: an up-to-date meta-analysis of RCTs applying the ISGPS (2016) criteria. Surg Laparosc Endosc Percutan Tech. 28:139–146. DOI: 10.1097/SLE.0000000000000530. PMID: 29683997. PMCID: PMC5999363.

16. Sauvanet A, Partensky C, Sastre B, Gigot JF, Fagniez PL, Tuech JJ, et al. 2002; Medial pancreatectomy: a multi-institutional retrospective study of 53 patients by the French Pancreas Club. Surgery. 132:836–843. DOI: 10.1067/msy.2002.127552. PMID: 12464868.

17. Wang ZZ, Zhao GD, Zhao ZM, Hu MG, Tan XL, Zhang X, et al. 2021; A comparative study of end-to-end pancreatic anastomosis versus pancreaticojejunostomy after robotic central pancreatectomy. Updates Surg. 73:967–975. DOI: 10.1007/s13304-021-01037-z. PMID: 33797734.

18. Regmi P, Yang Q, Hu HJ, Liu F, Karn HR, Ma WJ, et al. 2020; Overall postoperative morbidity and pancreatic fistula are relatively higher after central pancreatectomy than distal pancreatic resection: a systematic review and meta-analysis. Biomed Res Int. 2020:7038907. DOI: 10.1155/2020/7038907. PMID: 32219139. PMCID: PMC7057026.

19. Dragomir MP, Sabo AA, Petrescu GED, Li Y, Dumitrascu T. 2019; Central pancreatectomy: a comprehensive, up-to-date meta-analysis. Langenbecks Arch Surg. 404:945–958. DOI: 10.1007/s00423-019-01829-3. PMID: 31641855.

20. Zhang RC, Zhang B, Mou YP, Xu XW, Zhou YC, Huang CJ, et al. 2017; Comparison of clinical outcomes and quality of life between laparoscopic and open central pancreatectomy with pancreaticojejunostomy. Surg Endosc. 31:4756–4763. DOI: 10.1007/s00464-017-5552-7. PMID: 28424909.

Table 1
Comparison of clinical and demographic profiles between the two groups
| Variable | CP (n = 37) | DP | p-value | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Before matching (n = 51) | After matching (n = 37) | Before matching | After matching | |||
| Age (yr) | 43 (27–51) | 30 (23–46) | 30 (21.5–51.0) | 0.084 | 0.294 | |
| Female | 29 (78.4) | 32 (62.7) | 31 (83.8) | 0.116 | 0.553 | |
| Body mass index (kg/m2) | 20.7 (19.5–22.3) | 21.1 (19.8–22.1) | 21.1 (19.7–22.8) | 0.120 | 0.312 | |
| Comorbidities | 12 (32.4) | 11 (21.6) | 9 (24.3) | 0.252 | 0.439 | |
| ASA grade 1 | 37 (100) | 51 (100) | 37 (100) | |||
| Clinical features | ||||||
| Pain | 36 (97.3) | 49 (96.1) | 35 (94.6) | 0.756 | 0.556 | |
| Jaundice | 0 (0) | 0 (0) | 0 (0) | - | - | |
| Pancreatitis | 1 (2.7) | 1 (2.0) | 1 (2.7) | 0.818 | > 0.999 | |
| Weight loss | 2 (5.4) | 6 (11.8) | 4 (10.8) | 0.306 | 0.394 | |
| Endocrine insufficiency | 10 (27.0) | 8 (15.7) | 7 (18.9) | 0.193 | 0.407 | |
| Exocrine insufficiency | 2 (5.4) | 6 (11.8) | 5 (13.5) | 0.306 | 0.233 | |
| Biochemical parameters | ||||||
| Hemoglobin (g/dL) | 11 (10.7–12.2) | 12 (11.0–13.1) | 11.9 (11.0–13.0) | 0.324 | 0.098 | |
| TLC (cells/m3) | 7.3 (6.1–8.3) | 7.6 (6.2–8.4) | 7.4 (6.1–8.1) | 0.600 | 0.824 | |
| Platelets (cells/cumm) | 3.1 (2.2–3.7) | 3.0 (2.1–3.3) | 3.0 (2.0–3.2) | 0.198 | 0.168 | |
| Creatinine(mg/dL) | 1.0 (0.8–1.0) | 0.9 (0.8–1.0) | 1.0 (0.7–1.0) | 0.885 | 0.959 | |
| Bilirubin (mg/dL) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 0.119 | 0.092 | |
| Albumin (gm/dL) | 4.0 (3.8–4.0) | 3.9 (3.7–4.0) | 3.9 (3.7–4.0) | 0.029* | 0.041* | |
| CA 19.9 (IU/mL) | 12 (9.0–18.7) | 20 (14.0–23.0) | 21 (18.0–29.0) | 0.013* | 0.001* | |
| CEA (ng/mL) | 2.4 (2.0–3.0) | 2.5 (1.3–3.1) | 3.0 (1.9–3.8) | 0.922 | 0.358 | |
Table 2
Comparison of intraoperative variables between the two groups
| Variable | CP (n = 37) | DP | p-value | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Before matching (n = 51) | After Matching (n = 37) | Before matching | After matching | |||
| Duration of surgery (min) | 300 (292–330) | 270 (227–330) | 280 (250–330) | 0.565 | 0.147 | |
| Blood loss | 200 (137–250) | 150 (100–200) | 150 (150–200) | 0.127 | 0.010* | |
| Distal stump length | 5.0 (4.0–6.0) | 4 (4–4) | 4.0 (4.0–4.5) | < 0.001* | < 0.001* | |
| PD size (mm) | 2 (1–4) | 3 (2–3) | 3 (2–3) | 0.539 | 0.288 | |
| Distal stump anastomosis | ||||||
| Pancreatico jejunostomy | 33 (89.2) | |||||
| Pancreatico gastrostomy | 4 (10.8) | |||||
| Pancreas texture | ||||||
| Soft | 23 (62.2) | 34 (66.7) | 25 (67.6) | |||
| Hard | 04 (10.8) | 1 (2.0) | 1 (2.7) | 0.206 | 0.381 | |
| Unknown | 10 (27.0) | 16 (31.4) | 11 (29.7) | |||
| Type of Anastomosis | ||||||
| Duct to mucosa | 19 (51.4) | |||||
| Dunking | 18 (48.6) | |||||
| Histopathology | ||||||
| SPEN | 16 (43.2) | 24 (47.1) | 21 (56.8) | |||
| MCN | 3 (08.1) | 4 (7.8) | 4 (10.8) | |||
| SCN | 10 (27.0) | 7 (13.7) | 5 (13.5) | 0.715 | 0.292 | |
| NET | 6 (16.2) | 7 (13.7) | 2 (5.4) | |||
| Symptomatic simple cysts | 1 (2.7) | 4 (7.8) | 3 (8.1) | |||
| Pseudocysts | 0 (0) | 2 (3.9) | 1 (2.7) | |||
| GIST | 0 (0) | 1 (2.0) | 0 (0) | |||
| Adenocarcinoma | 1 (2.7) | 1 (2.0) | 1 (2.7) | |||
| Papillary cystadenocarcinoma | 0 (0) | 1 (2.0) | 0 (0) | |||
| Size of lesion (cm) | 4.0 (3.0–5.1) | 7 (5.0–9.0) | 7 (5.0–9.0) | < 0.001* | < 0.001* | |
Table 3
Comparison of complications between the two groups
| Variable | CP (n = 37) | DP | p-value | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Before matching (n = 51) | After matching (n = 37) | Before matching | After matching | |||
| Clavien-Dindo morbidity | ||||||
| Grade 1 | 9 (24.3) | 9 (17.6) | 7 (18.9) | |||
| Grade 2 | 5 (13.5) | 3 (5.9) | 1 (2.7) | |||
| Grade 3 | 2 (5.4) | 2 (3.9) | 2 (5.4) | 0.461 | 0.277 | |
| Grade 4 | 1 (2.7) | 1 (2.0) | 0 (0) | |||
| Grade 5 | 1 (2.7) | 0 (0) | 0 (0) | |||
| Grade 3 or above complication | 4 (10.8) | 3 (5.9) | 2 (5.4) | 0.182 | 0.158 | |
| Readmission in 30 days | 1 (2.7) | 0 (0) | 0 (0) | 0.238 | 0.314 | |
| POPF | 16 (43.2) | 13 (25.5) | 8 (21.6) | 0.080 | 0.047* | |
| POPF* | ||||||
| Grade A | 11 (29.7) | 13 (25.5) | 8 (21.6) | |||
| Grade B | 2 (5.4) | 1 (2.0) | 0 (0) | 0.120 | 0.186 | |
| Grade C | 3 (8.1) | 0 (0) | 0 (0) | |||
| Grade B & C | 5 (13.5) | 1 (2.0) | 0 (0) | 0.078 | 0.054 | |
| Postoperative bleeding | 1 (2.7) | 0 (0) | 0 (0) | 0.238 | 0.314 | |
| Delayed gastric emptying | 12 (32.4) | 7 (13.7) | 7 (18.9) | 0.035* | 0.183 | |
| Surgical site infection | 10 (27.0) | 9 (17.6) | 8 (21.6) | 0.291 | 0.588 | |
| Pulmonary complication | 2 (5.4) | 1 (2.0) | 1 (2.7) | 0.379 | 0.556 | |
| Postoperative hospital stay | 10 (7–18) | 8 (7–11) | 8 (7–9) | 0.319 | 0.128 | |
| Overall morbidities | 18 (48.6) | 15 (29.4) | 10 (27.0) | 0.123 | 0.092 | |
Table 4
Comparison of long-term outcomes between the two groups
| Variable | CP (n = 37) | DP | p-value | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Before matching (n = 51) | After matching (n = 37) | Before matching | After matching | |||
| Follow up (mon) | 22 (11–118) | 81 (18–117) | 63 (18–117) | 0.095 | 0.071 | |
| Endocrine insufficiency | ||||||
| Absent | 26 (70.3) | 37 (72.5) | 25 (67.6) | |||
| Unchanged | 10 (27.0) | 5 (9.8) | 4 (10.8) | 0.041* | 0.041* | |
| Worsening | 0 (0) | 4 (7.8) | 3 (8.1) | |||
| New-onset | 1 (2.7) | 5 (9.8) | 5 (13.5) | |||
| Exocrine insufficiency | ||||||
| Absent | 26 (70.3) | 32 (62.7) | 23 (62.2) | |||
| Unchanged | 2 (5.4) | 4 (7.8) | 3 (8.1) | |||
| Worsening | 8 (21.6) | 2 (3.9) | 1 (2.7) | 0.164 | 0.153 | |
| New-onset | 1 (2.7) | 2 (3.9) | 2 (5.4) | |||
| Weight gain | ||||||
| Same | 17 (45.9) | 13 (25.4) | 8 (21.6) | |||
| Present | 17 (45.9) | 10 (19.6) | 8 (21.6) | 0.016* | 0.021* | |
| Lost | 1 (2.7) | 4 (7.8) | 4 (10.8) | |||
| SF-36 | ||||||
| Physical function | 26.3 (95.0) | 26.5 (85.0) | 22.9 (85.0) | 0.962 | 0.972 | |
| Physical health | 29.2 (100.0) | 24.3 (100.0) | 20.3 (100.0) | 0.089 | 0.055 | |
| Emotional Problem | 29.2 (100.0) | 24.3 (100.0) | 20.3 (100.0) | 0.089 | 0.055 | |
| Energy/fatigue | 29.5 (75.0) | 24.1 (60.0) | 20.8 (60.0) | 0.188 | 0.271 | |
| Emotional well being | 27.5 (80.0) | 25.6 (92.0) | 20.5 (68.0) | 0.662 | 0.208 | |
| Social functioning | 28.6 (87.5) | 24.8 (87.5) | 21.2 (87.5) | 0.313 | 0.322 | |
| Pain | 31.7 (100.0) | 22.3 (100.0) | 19.6 (100.0) | 0.012* | 0.044* | |
| General health | 31.8 (50.0) | 22.2 (50.0) | 19.0 (60.0) | 0.021* | 0.049* | |
| Health Change | 27.4 (50.0) | 25.7 (50.0) | 22.9 (50.0) | 0.646 | 0.971 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download