Abstract
Backgrounds/Aims
The aim of this study was to describe short- and long-term outcomes of patients who underwent pancreatoduodenectomy (PD) at a typical United Kingdom hepatopancreatobiliary unit.
Methods
A retrospective analysis of all PD patients with histologically-confirmed pancreatic ductal adenocarcinoma (PDAC), ampullary adenocarcinoma (AA), or distal cholangiocarcinoma (CC) from September 1st, 2006 to May 31st, 2015 was carried out. The following information was obtained: demographics, comorbidities, preoperative investigations, neoadjuvant treatment, operative details, postoperative management, complications, adjuvant treatment, five-year recurrence, and five-year survival. Effects of selected preoperative variables on short- and long-term outcomes were investigated.
Results
Of 271 included patients, 57.9% had PDAC, 25.8% had AA, and 16.2% had CC. In total, 67.9% experienced morbidity and 17.3% developed a Clavien-Dindo grade ≥ III complication. The 90-day mortality was 3.3%. Clinically-relevant postoperative pancreatic fistula, bile leak, gastrojejunal leak, postpancreatectomy haemorrhage and delayed gastric emptying affected 8.1%, 4.1%, 0.0%, 9.2%, and 19.9% of patients, respectively. American Society of Anesthesiologists grade III–VI correlated with overall morbidity (p = 0.002) and major morbidity (p = 0.009), but not 90-day mortality or five-year survival. The same pattern was observed in patients with a preoperative serum bilirubin > 29 μmol/L and/or a neutrophil/lymphocyte ratio > 3.1. Five-year cancer recurrence and five-year survival were 68.3% and 22.5%, respectively. PDAC patients had higher five-year recurrence but lower five-year survival rates (both p = 0.001).
Pancreatic ductal adenocarcinoma (PDAC) of the pancreatic head is a leading cause of cancer-related death in the Western world. Its incidence is set to increase across the globe [1]. Unfortunately, most patients (up to 80%) present with metastatic disease and prognosis is very poor [1]. Although ampullary adenocarcinoma (AA) and distal cholangiocarcinoma (CC) are less common than PDAC, their prognoses are only marginally better [2,3]. Patients with one of these cancers may undergo pancreaticoduodenectomy (PD) providing they present with resectable disease and have an appropriate performance status. Whilst this offers some patients (up to 20%) the possibility of long-term survival, overall morbidity is often quoted at 50% and rates of early recurrence are high [4,5]. The aim of this study was to describe the experience of a typical tertiary hepatopancreatobiliary (HPB) unit in the United Kingdom (UK) by compiling a PD complication profile and investigating effects of selected variables on short- and long-term outcomes.
This study adhered to the standards laid down in the Declaration of Helsinki (revised 2013). It was approved by North West - Greater Manchester South Research Ethics Committee (20/NW/0397) as part of the Recurrence After Whipple’s (RAW) study (IRAS ID: 280423) and our own research and development department. Patients were included if they underwent PD at our centre between September 1st, 2006 and May 31st, 2015. In all cases, the procedure was performed or supervised by a consultant HPB surgeon. An attempt was made to include all eligible patients since our HPB unit was formally established. The end date of May 31st, 2015 was chosen to complete five-year follow-up for all included patients. Patients were excluded if they were lost to follow-up before the five-year follow-up or if their medical notes were lost, destroyed or unavailable. Potentially eligible patients were screened from a prospectively maintained departmental database. Eligibility was confirmed and data were collected from physical and electronic patient records. If follow-up data were not available locally, this was collected from referring hospitals. A purpose-built electronic database was created using REDCap (v11.0.3; Vanderbilt University, Nashville, TN, USA) to collect information on the following: demographics, comorbidities, preoperative investigations, neoadjuvant treatment (if given), operative details, postoperative management and complications, histology results, adjuvant treatment (if given), cancer recurrence, palliative treatment (if given), and five-year survival.
Definitions of complications are shown in Supplementary Table 1–5. Postoperative pancreatic fistula (POPF) was categorised as biochemical leak (formerly grade A POPF) or clinically-relevant (CR)-POPF (grade B or grade C POPF) according to the International Study Group of Pancreatic Surgery 2016 definitions [6]. Bile leak was categorised as grades A, B, and C per the International Study Group for Liver Surgery (ISGLS) 2011 definitions [7]. Postpancreatectomy haemorrhage (PPH) [8] and delayed gastric emptying (DGE) [9] were defined as grades A, B and C per ISGPS 2007 definitions. Patients were considered to have had a chest infection if they were given antibiotics during their index admission for a clinically or radiologically diagnosed chest infection. Intra-abdominal collection was radiologically diagnosed (usually by computed tomography). Surgical site infection was clinically diagnosed. All complications were graded using the Clavien-Dindo (CD) classification of surgical complications [10]. If not confirmed radiologically, cancer recurrence was assumed if a patient had a raised CA 19-9 with relevant signs/symptoms. Patients were deemed to have had a cardiovascular comorbidity if any of the following had been previously diagnosed: hypertension, atrial fibrillation (AF), cardiac arrythmia (other than AF), ischaemic heart disease, heart failure, peripheral vascular disease, previous stroke or previous transient ischaemic attack. Patients were deemed to have had a respiratory comorbidity if any of the following had been previously diagnosed: asthma, chronic obstructive pulmonary disease, pulmonary fibrosis or pulmonary embolism. Concerning postoperative histology, a positive resection margin (R1) included any resection margin where tumour cells were visible within 1 mm of the margin. A negative resection margin (R0) included all margins where no cancer cells were visible at the margin or within 1 mm of the margin.
Categorical data are presented as frequency counts and associated percentages. Continuous data are presented as medians with range. When comparing patients by their histological diagnosis, medians were compared using the Kruskal–Wallis test. Other variables were compared using Fisher’s exact test. The latter was also used to investigate correlations of selected preoperative variables (age, body mass index [BMI], comorbidities, American Society of Anesthesiologists [ASA] grade, serum albumin, serum bilirubin and neutrophil/lymphocyte ratio [NLR]) with overall morbidity, major morbidity (CD grade I–II complications excluded), 90-day mortality and five-year survival. These variables were selected as this information for collected as part of the RAW study. The Kaplan–Meier method was used to compare survival among patients with PDAC, AA and distal CC (patients with intra- or hilar CC were not included). The Mantel-Cox method was used to test for statistical significance. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using Microsoft Excel (v2013; Microsoft Corp., Redmond, WA, USA), GraphPad Prism (v9.3.1; GraphPad software, San Diego, CA, USA) and IBM SPSS Statistics (v2015; IBM Corp., Armonk, NY, USA).
In total, 351 records were screened for eligibility. Eighty patients were excluded as they did not meet the inclusion criteria (Fig. 1). The final analysis included 271 patients. Of these patients, 157 (57.9%) had a postoperative histological diagnosis of PDAC, 70 (25.8%) had an AA, and 44 (16.2%) had a distal CC (Table 1). The median age was 66 years (range: 33–83 years). The median BMI was 25.9 kg/m2 (range: 16.4–53.4 kg/m2). Concerning comorbidities, 42 (15.5%) patients had a prior history of diabetes, 104 (38.4%) had a cardiovascular comorbidity, and 46 (17.0%) had a respiratory comorbidity. Diabetes was significantly more common in patients with PDAC (p = 0.013). Respiratory comorbidities were more common in those with a CC (p = 0.048). A total of 28 (10.3%) patients had a prior history of cancer (excluding those being treated with PD). The decision was made to include these patients in the analyses as none died secondary to recurrence of their non-PD-related cancer. In addition, five-year survival rates were similar between those who had a prior cancer and those who did not (21.4% vs. 22.6%, p = 1.000). A total of 221 (81.5%) patients had received a preoperative biliary stent. Very few patients received neoadjuvant chemotherapy (1.1%) or radiotherapy (0.8%). A majority (65.3%) of patients were ASA grade I–II. Of all patients, 72.0% received a pancreato-gastrostomy. A total of 35 (12.9%) patients underwent concomitant venous resection and seven (2.6%) underwent concomitant arterial resection. Venous resection was significantly more common in patients with PDAC (p < 0.001).
The median length of stay was 11 days (range: 3–102 days). A total of 18 (6.6%) patients were readmitted to hospital within 30 days of discharge. Nine (3.3%) patients died within 90 days of their index procedure. Two patients developed early disease recurrence and died with disseminated disease. One died of intra-abdominal sepsis. One died with gastrointestinal haemorrhage. One died secondary to a splenic artery haemorrhage. One patient died secondary to hospital acquired pneumonia. One patient developed multiorgan failure secondary to faecal peritonitis caused by a stercoral perforation. For the remaining two cases, the cause of death was unclear. Both patients underwent a post-mortem examination. In one, the patient was found to have an infarcted liver without other significant findings. In the other case, the cause of death was not identified.
The median tumour size was 30 mm (range: 5–130 mm). Patients with PDAC had significantly larger tumours (p < 0.001). Concerning resection margins, 101 (37.3%) patients had no positive margins (R0), 166 (61.3%) had at least one positive margin (R1) and four (1.5%) patients had an incomplete (R2) resection. An R0 resection was the most common in those with AA (p < 0.001). An R1 resection was most common in patients with PDAC (p < 0.001). The median number of resected nodes was 16 (range: 1–38). The median number of involved nodes was 2 (range: 0–21). The number of involved nodes was significantly higher in patients with PDAC (p < 0.001).
One hundred and eighty-four (67.9%) patients experienced at least one complication and 47 (17.3%) experienced a CD grade ≥ III complication. A total of 401 postoperative complications were recorded (Table 2). Of them, 109 (27.2%) were CD grade I, 214 (53.2%) were grade II, 52 (12.7%) were grade III, 18 (4.4%) were grade IV and six (1.5%) were grade V. CR-POPF affected 22 (8.1%) cases (17 grade B and five grade C). Bile leak affected 11 (4.1%) cases (six grade A and five grade B). No patients experienced a gastrointestinal leak. PPH affected 25 (9.2%) patients (three grade A, 13 grade B and ten grade C). Other commonly occurring complications included surgical site infection (21.8%), chest infection (16.6%), intra-abdominal collection (13.3%) and ileus (9.6%). Other complications of note included cardiac arrythmia (5.9%) and chyle leak (4.3%).
When patients aged ≥ 66 years (median age) were compared to those aged < 66 years, there was no significant difference in overall morbidity (69.1% vs. 66.7%, p = 0.698), major morbidity (17.3% vs. 17.4%, p > 0.999) or 90-day mortality (2.2% vs. 4.5%, p = 0.325) (Table 3). The median was used as the cut-off so that two equal sized groups could be compared. The same pattern was observed concerning preoperative BMI and serum albumin. Patients with a preoperative bilirubin ≤ 29 µmol/L less often experienced morbidity (61.6% vs. 74.4%, p = 0.027) and major morbidity (11.6% vs. 23.3%, p = 0.016). However, a preoperative bilirubin ≤ 29 µmol/L did not affect 90-day mortality (2.9% vs. 3.8%, p = 0.746). Similarly, those with a preoperative neutrophil/lymphocyte ratio (NLR) ≤ 3.1 had lower rates of morbidity (61.8% vs. 74.1%, p = 0.037) and major morbidity (11.2% vs. 23.7%, p = 0.006), but the difference in 90-day mortality was not significant (2.2% vs. 4.4%, p = 0.334). Patients with a preoperative diagnosis of diabetes mellitus (DM) or cardiorespiratory disease had similar overall morbidity (70.7% vs. 64.5%, p = 0.297), major morbidity (22.3% vs. 13.3%, p = 0.055) and 90-day mortality (2.7% vs. 4.1%, p = 0.519) to those without these conditions. In contrast, an ASA grade of III–IV correlated with increased overall morbidity (81.2% vs. 62.1%, p = 0.002) and major morbidity (27.1% vs. 13.0%, p = 0.009), although the difference in 90-day mortality was not significant (5.9% vs. 2.3%, p = 0.155).
A total of 151 (57.2%) patients received adjuvant chemotherapy (Table 4). The median number of cycles was six (range: 1–12). Of those who commenced adjuvant chemotherapy, 75.8% completed the planned course. Five-year cancer recurrence affected 68.3% of patients. Recurrence was significantly more frequent among patients with PDAC than in those with AA (77.1% vs. 55.7%, p < 0.001). Among patients who developed recurrent disease, the median time to diagnosis was nine months (range: 0–58 months). This was shortest among those with PDAC (p = 0.02). Palliative chemotherapy was received by 31.4% of patients that developed recurrent disease. Overall, five-year survival was 22.5%. Five-year survival was lowest in PDAC patients (14.0% vs. 40.0%, p = 0.001) and was not significantly affected by age, BMI, preoperative comorbidities, ASA grade or preoperative blood tests (Table 3). Estimated median time to recurrence (p = 0.049) and estimated median overall survival (p = 0.001) were significantly lower in PDAC patients (Fig. 2).
This article describes the short- and long-term outcomes of 271 patients who underwent PD for histologically-confirmed PDAC, AA or CC at a typical tertiary HPB unit in the UK between September 2006 and May 2015 (inclusive). Few prior articles have reported both surgical and long-term outcomes. Our study could be compared to that of El Nakeeb et al. [11] who studied PD cancer patients at an Egyptian centre between 1993 and 2017. The median age was considerably higher in our study (66 vs. 54 years), which might reflect the more elderly population of the UK. However, numbers of patients with preoperative DM were similar (15.5% vs. 14.5%). In our study, 81.5% of patients underwent preoperative biliary drainage (vs. 51.1% in [11]). The median preoperative serum albumin was the same for both studies (40 g/L). However, the median bilirubin was higher in the Egyptian study (40 µmol/L vs. 29 µmol/L). Although similar numbers of patients received a pancreato-gastrostomy in the two studies, a considerably higher proportion of patients underwent a vascular resection in our study (12.9% vs. 1.2%). The median tumour size was the same in both studies. However, the median length of stay was considerably longer in our study (11 days vs. 8 days). This might be because healthcare is publicly funded in the UK. In our study, 8.1% of patients developed a CR-POPF (vs. 7.2% in [11]), 19.9% developed DGE (vs. 18.0% in [11]) and 7.3% developed a bile leak (vs. 4.1% in [11]). A similar number of patients in each study had an unplanned return to theatre and five-year survival rates were also similar.
The incidence of CR-POPF was slightly lower in our study than in a recent systematic review (10.0%–25.9%) [12]. This could be partly explained by the high proportion of patients who received a pancreato-gastrostomy. However, a recent randomised controlled trial did not suggest that pancreato-gastrostomy was more protective than pancreato-jejunostomy [13]. Our observed incidence rates for bile leak, PPH, cholangitis, chyle leak and DGE were similar to those described in the literature [12]. No patients in our study developed a gastrojejunal anastomotic leak. A recent systematic review suggested that this complication affects 0.4%–1.2% of PD patients [12].
Prior studies have suggested that advanced age alone should not be an absolute contraindication to PD [17,18]. Whilst some authors have suggested older patients are at increased risk of morbidity [14-16], others have shown that selected older patients have similar perioperative and survival outcomes to younger patients [17,18]. Our findings showed that older patients had similar overall morbidity, major morbidity and five-year survival to younger patients. Although the 90-day mortality was slightly higher in older patients, this difference was not significant. Whilst older patients should not be discriminated against if they are fit, they might be less inclined to opt for surgical management as there might be less of a perceived gain. Additionally, previous studies have demonstrated that favourable outcomes in the elderly might have been affected by selection bias [17,18].
Obesity is associated with poor operative outcomes for a number of reasons. However, a high BMI should not be a contraindication to resection. Obese patients tend to have a reduced residual capacity and are high risk for atelectasis and shunting [19]. These patients also have a high resting metabolic rate, work of breathing and minute oxygen demand [19]. In addition, being overweight is often associated with hypertension, increased cardiac workload and a prothrombotic state [20]. Finally, from a surgical point of view, access can be difficult and a high amount of intra-abdominal adipose tissue can cause further challenges. However, in our series, being overweight did not appear to correlate with adverse outcomes. This finding was unexpected. It was not consistent with the results of other similar studies. Chen et al. [21] suggested that a BMI > 24 kg/m2 results in an increased risk of perioperative morbidity. Aoki et al. [22] suggested a BMI > 25 kg/m2 correlates with grade C POPF and major morbidity. El Nakeeb et al. [23] found BMI > 25 kg/m2 was associated with increased overall morbidity and perioperative mortality [24,25]. Del Chiaro et al. [26] also found that a BMI > 25 kg/m2 is associated with increased intra-operative blood loss and increased risk of POPF. Greenblatt et al. [27] concluded that a BMI > 25 kg/m2 is a predictor of overall morbidity, but not perioperative mortality. Interestingly, some studies have shown that obese patients might have an advantage when it comes to long-term outcomes. Tsai et al. [28] have suggested that overweight and obese patients show better five-year survival than patients with a healthy weight. However, other similar studies have not observed this.
In our study, patients with a preoperative comorbidity (DM, cardiovascular or respiratory disease) had similar short- and long-term outcomes to those without these conditions. The impact of DM on PD outcomes remains controversial. Deo et al. [29] have found that preoperative DM does not affect surgical outcomes, although five-year survival is lower among diabetics. Since patients with diabetics are thought to have a soft pancreas with a high fat content (both risk factors for POPF) [29], it has been suggested they have a higher risk of developing POPF. However, two recent meta-analyses have disputed this [30,31]. Other studies have suggested that patients with diabetes are high risk for developing delayed gastric emptying due to vagal neuropathy and hyperglycaemia-induced reduction of gastric emptying time [32], although this is also controversial. Additionally, since long-term hyperglycaemia is thought to impair immune function, some authors have suggested diabetics are at increased risk of infective complications [33]. A recent meta-analysis [30] has suggested this is not the case. The conclusions of this meta-analysis might reflect the greater degree of care often shown for patients who are perceived to be at increased risk (e.g., a surgeon may subconsciously pay more attention during a high-risk case or put pressure on the intensive care unit to keep hold of a patient rather than discharge them).
The impact of pre-existing cardiac disease on PD outcomes is more clear-cut. Ronnekleiv-Kelly et al. [34] have found that patients with a cardiac comorbidity are at an increased risk of cardiac complications, major morbidity and mortality. Other authors have reached similar conclusions [35,36]. To the best of our knowledge, no studies have specifically investigated whether cardiac disease affects long-term survival. Very few studies have investigated the impact of pre-existing respiratory comorbidities on PD outcomes. This is likely because few patients with a significant respiratory comorbidity would be offered a resection. Shia et al. [37] have found that patients with chronic obstructive pulmonary disease have reduced 90-day survival. Aoki et al. [22] have found that patients with respiratory disease have higher rates of major morbidity and POPF compared to patients without these comorbidities.
In our study, ASA grade I–II patients were significantly less likely to experience morbidity or major morbidity. However, these patients had similar 90-day mortality and five-year survival rates to those with a high ASA grade. Similar findings have been reported in the literature. Eeson et al. [38] have found that ASA grade III patients have an increased risk of perioperative mortality. However, this was not significant once age was adjusted for. Whilst this study did not look at five-year survival, ASA grade III patients had reduced median overall survival than those with an ASA grade of I or II [38]. Other authors have also found that increasing ASA grade is correlated with additional morbidity risk [39,40].
We found that patients with high preoperative serum bilirubin levels more often experienced morbidity or major morbidity. However, this did not affect 90-day mortality or five-year survival. Scheufele et al. [41] have found that bilirubin level does not affect overall morbidity or long-term survival. Pamecha et al. [42] reached similar conclusions, although severely jaundiced patients had increased intraoperative blood loss. Wang et al. [43] have also found that bilirubin level does not affect long-term outcomes, although severely jaundiced patients have higher rates of infective complications. A number of theories have been put forward to try and explain why this might be. Firstly, biliary stasis favours microbial proliferation in a normally sterile site. In addition, increased pressure within the biliary tree can lead to retrograde flow of bile and provide a route for organisms to enter the systemic circulation [44]. Furthermore, the synthetic function of hepatocytes may be affected, resulting in impaired immune function [44].
Neutrophils are the most abundant type of lymphocytes. Neutrophilia has long been associated with poor outcomes for cancer patients. It is thought that neutrophilia along with sustained inflammation may promote angiogenesis, tumorigenesis and metastasis, thus protecting cancer cells from immune-mediated destruction [45]. Lymphopenia occurs in many types of cancer. It is associated with an immunocompromised state. It is thought to correlate with poor outcomes due to an impaired response to tumour cells and an increased risk of infective complications [46]. Whilst the mechanism behind this impaired response is poorly understood, a high NLR has been shown to correlate with poor short- and long-term PD outcomes [47,48], although the clinical implications of a high NLR are currently unknown. Our results showed that patients with a NLR > 3.1 more often experienced morbidity and major morbidity. However, a NLR > 3.1 did not affect 90-day mortality or five-year survival. Other authors have also observed this. Arikan et al. [47] have found that those with a high NLR show increased morbidity rates and are more likely to develop POPF. Other authors have reached similar conclusions [49-51]. Unlike in our study, some prior studies have shown that a high NLR is associated with reduced overall survival [48]. We did not observe this. This may be because of the low number of patients that achieved five-year survival.
Our study has several limitations. It was a single centre, retrospective study with a relatively small sample size. Additionally, due to a long study period (some cases were performed as far back as 2006), practice likely changed significantly during the study window. This fact, along with the definitions used might help explain the relatively high number of PDAC patients with a positive resection margin status. Patient selection and surgical techniques have likely improved considerably since 2006. When investigating the impact of the selected variables on perioperative and long-term outcomes, we were not able to consider all relevant variables or the impact of potential confounding factors. The selected variables were chosen as information on these were collected as part of the RAW study. Thus, other important factors were not considered. We were unable to perform independent analyses due to the small sample size. Our analysis was also affected by the fact that a relatively low number of patients achieved five-year survival. However, our dataset is robust and few other studies have reported on both surgical and five-year outcomes.
In our series, most PD patients developed at least one complication. However, few experienced major morbidity. Rates of CR-POPF, bile leak, gastrojejunal leak, PPH and DGE were 8.1%, 4.1%, 0.0%, 9.2%, and 19.9%, respectively. ASA grade III–IV patients and those with a high preoperative bilirubin and/or NLR more often experienced morbidity and/or major morbidity. Five-year recurrence and survival rates were 68.3% and 22.5%, respectively. The preoperative variables analysed in this study did not affect five-year survival. Surgeons who perform PD should have a sound understanding of figures quoted to guide patient selection and the consenting process.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.22-039.
Notes
AUTHOR CONTRIBUTIONS
Conceptualisation: All authors. Data curation: All authors. Formal analysis: All authors. Investigation: All authors. Methodology: All authors. Project administration: TBR, PLZL. Resources: All authors. Software: All authors. Supervision: SA. Validation: SA. Visualisation: All authors. Writing - original draft: TBR. Writing - review and editing: All authors.
REFERENCES
1. Bekkali NLH, Oppong KW. 2017; Pancreatic ductal adenocarcinoma epidemiology and risk assessment: could we prevent? Possibility for an early diagnosis. Endosc Ultrasound. 6(Suppl 3):S58–S61. DOI: 10.4103/eus.eus_60_17. PMID: 29387690. PMCID: PMC5774073.

2. Ahn DH, Bekaii-Saab T. 2014; Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book. 34:112–115. DOI: 10.14694/EdBook_AM.2014.34.112. PMID: 24857067. PMCID: PMC4966534.

3. Lessing Y, Pencovich N, Nevo N, Lubezky N, Goykhman Y, Nakache R, et al. 2019; Early reoperation following pancreaticoduodenectomy: impact on morbidity, mortality, and long-term survival. World J Surg Oncol. 17:26. DOI: 10.1186/s12957-019-1569-9. PMID: 30704497. PMCID: PMC6357503.

4. Crippa S, Belfiori G, Bissolati M, Partelli S, Pagnanelli M, Tamburrino D, et al. 2021; Recurrence after surgical resection of pancreatic cancer: the importance of postoperative complications beyond tumor biology. HPB (Oxford). 23:1666–1673. DOI: 10.1016/j.hpb.2021.04.004. PMID: 33934960.

5. Pugalenthi A, Protic M, Gonen M, Kingham TP, Angelica MI, Dematteo RP, et al. 2016; Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Surg Oncol. 113:188–193. DOI: 10.1002/jso.24125. PMID: 26678349. PMCID: PMC4830358.

6. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. 2017; The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 161:584–591. DOI: 10.1016/j.surg.2016.11.014. PMID: 28040257.

7. Brooke-Smith M, Figueras J, Ullah S, Rees M, Vauthey JN, Hugh TJ, et al. 2015; Prospective evaluation of the International Study Group for Liver Surgery definition of bile leak after a liver resection and the role of routine operative drainage: an international multicentre study. HPB (Oxford). 17:46–51. DOI: 10.1111/hpb.12322. PMID: 25059275. PMCID: PMC4266440.

8. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. 2007; Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 142:20–25. DOI: 10.1016/j.surg.2007.02.001. PMID: 17629996.

9. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. 2007; Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 142:761–768. DOI: 10.1016/j.surg.2007.05.005. PMID: 17981197.

10. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. 2009; The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 250:187–196. DOI: 10.1097/SLA.0b013e3181b13ca2. PMID: 19638912.
11. El Nakeeb A, Askar W, Atef E, Hanafy EE, Sultan AM, Salah T, et al. 2017; Trends and outcomes of pancreaticoduodenectomy for periampullary tumors: a 25-year single-center study of 1000 consecutive cases. World J Gastroenterol. 23:7025–7036. DOI: 10.3748/wjg.v23.i38.7025. PMID: 29097875. PMCID: PMC5658320.

12. Russell TB, Aroori S. 2022; Procedure-specific morbidity of pancreatoduodenectomy: a systematic review of incidence and risk factors. ANZ J Surg. 92:1347–1355. DOI: 10.1111/ans.17473. PMID: 35088514.

13. Keck T, Wellner UF, Bahra M, Klein F, Sick O, Niedergethmann M, et al. 2016; Pancreatogastrostomy versus pancreatojejunostomy for RECOnstruction after PANCreatoduodenectomy (RECOPANC, DRKS 00000767): perioperative and long-term results of a multicenter randomized controlled trial. Ann Surg. 263:440–449. DOI: 10.1097/SLA.0000000000001240. PMID: 26135690. PMCID: PMC4741417.

14. El Nakeeb A, Atef E, El Hanafy E, Salem A, Askar W, Ezzat H, et al. 2016; Outcomes of pancreaticoduodenectomy in elderly patients. Hepatobiliary Pancreat Dis Int. 15:419–427. DOI: 10.1016/S1499-3872(16)60105-4. PMID: 27498583.

15. Zhang D, Gao J, Li S, Wang F, Zhu J, Leng X. 2015; Outcome after pancreaticoduodenectomy for malignancy in elderly patients. Hepatogastroenterology. 62:451–454.
16. Wiltberger G, Muhl B, Benzing C, Hau HM, Bartels M, Krenzien F. 2017; Pancreaticoduodenectomy in the elderly patient: age-adapted risk assessment. Dig Surg. 34:43–51. DOI: 10.1159/000448059. PMID: 27434057.

17. Shamali A, DéAth HD, Jaber B, Abuawad M, Barbaro S, Hamaday Z, et al. 2017; Elderly patients have similar short term outcomes and five-year survival compared to younger patients after pancreaticoduodenectomy. Int J Surg. 45:138–143. DOI: 10.1016/j.ijsu.2017.07.106. PMID: 28782662.

18. Gruppo M, Tolin F, Franzato B, Pilati P, Spolverato YC, Zingales F, et al. 2020; Impact of age on short- and long-term outcomes after pancreatoduodenectomy for periampullary neoplasms. Gastroenterol Res Pract. 2020:1793051. DOI: 10.1155/2020/1793051. PMID: 32382261. PMCID: PMC7183022.

19. Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, et al. 1998; The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 87:654–660. DOI: 10.1213/00000539-199809000-00031. PMID: 9728848.

20. Nightingale CE, Margarson MP, Shearer E, Redman JW, Lucas DN, et al. Members of the Working Party. Peri-operative management of the obese surgical patient 2015: Association of Anaesthetists of Great Britain and Ireland Society for Obesity and Bariatric Anaesthesia. Anaesthesia. 2015; 70:859–876. DOI: 10.1111/anae.13101. PMID: 25950621. PMCID: PMC5029585.
21. Chen YT, Deng Q, Che X, Zhang JW, Chen YH, Zhao DB, et al. 2015; Impact of body mass index on complications following pancreatectomy: ten-year experience at National Cancer Center in China. World J Gastroenterol. 21:7218–7224. DOI: 10.3748/wjg.v21.i23.7218. PMID: 26109808. PMCID: PMC4476883.

22. Aoki S, Miyata H, Konno H, Gotoh M, Motoi F, Kumamaru H, et al. 2017; Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: a nationwide study of 17,564 patients in Japan. J Hepatobiliary Pancreat Sci. 24:243–251. DOI: 10.1002/jhbp.438. PMID: 28196308. PMCID: PMC5516144.

23. El Nakeeb A, Hamed H, Shehta A, Askr W, El Dosoky M, Said R, et al. 2014; Impact of obesity on surgical outcomes post-pancreaticoduodenectomy: a case-control study. Int J Surg. 12:488–493. DOI: 10.1016/j.ijsu.2014.01.017. PMID: 24486933.

24. Dandona M, Linehan D, Hawkins W, Strasberg S, Gao F, Wang-Gillam A. 2011; Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas. 40:931–937. DOI: 10.1097/MPA.0b013e318215a9b1. PMID: 21747317.

25. Gaujoux S, Torres J, Olson S, Winston C, Gonen M, Brennan MF, et al. 2012; Impact of obesity and body fat distribution on survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg Oncol. 19:2908–2916. DOI: 10.1245/s10434-012-2301-y. PMID: 22411205.

26. Del Chiaro M, Rangelova E, Ansorge C, Blomberg J, Segersvärd R. 2013; Impact of body mass index for patients undergoing pancreaticoduodenectomy. World J Gastrointest Pathophysiol. 4:37–42. DOI: 10.4291/wjgp.v4.i2.37. PMID: 23755369. PMCID: PMC3676538.

27. Greenblatt DY, Kelly KJ, Rajamanickam V, Wan Y, Hanson T, Rettammel R, et al. 2011; Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 18:2126–2135. DOI: 10.1245/s10434-011-1594-6. PMID: 21336514.

28. Tsai S, Choti MA, Assumpcao L, Cameron JL, Gleisner AL, Herman JM, et al. 2010; Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg. 14:1143–1150. DOI: 10.1007/s11605-010-1201-3. PMID: 20431978.

29. Deo KB, Kulkarni AA, Kumar-M P, Krishnamurthy G, Shenvi S, Rana SS, et al. 2021; Impact of diabetes mellitus on morbidity and survival after pancreaticoduodenectomy for malignancy. Ann Hepatobiliary Pancreat Surg. 25:230–241. DOI: 10.14701/ahbps.2021.25.2.230. PMID: 34053926. PMCID: PMC8180397.

30. Lv X, Qiao W, Leng Y, Wu L, Zhou Y. 2017; Impact of diabetes mellitus on clinical outcomes of pancreatic cancer after surgical resection: a systematic review and meta-analysis. PLoS One. 12:e0171370. DOI: 10.1371/journal.pone.0171370. PMID: 28158300. PMCID: PMC5291503.

31. Xia X, Huang C, Cen G, Qiu ZJ. 2015; Preoperative diabetes as a protective factor for pancreatic fistula after pancreaticoduodenectomy: a meta-analysis. Hepatobiliary Pancreat Dis Int. 14:132–138. DOI: 10.1016/S1499-3872(15)60330-7. PMID: 25865684.

32. Nakeeb AE, Shehta A, Said R, Dosoky ME, Moneer A, Elrefai M, et al. 2018; Impact of diabetes mellitus on the outcomes after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a single center experience. Surg Gastroenterol Oncol. 23:342–349. DOI: 10.21614/sgo-23-5-342.

33. King JT Jr, Goulet JL, Perkal MF, Rosenthal RA. 2011; Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg. 253:158–165. DOI: 10.1097/SLA.0b013e3181f9bb3a. PMID: 21135698.

34. Ronnekleiv-Kelly SM, Greenblatt DY, Lin CP, Kelly KJ, Cho CS, Winslow ER, et al. 2014; Impact of cardiac comorbidity on early outcomes after pancreatic resection. J Gastrointest Surg. 18:512–522. DOI: 10.1007/s11605-013-2399-7. PMID: 24277570. PMCID: PMC4804699.

35. de la Fuente SG, Bennett KM, Pappas TN, Scarborough JE. 2011; Pre- and intraoperative variables affecting early outcomes in elderly patients undergoing pancreaticoduodenectomy. HPB (Oxford). 13:887–892. DOI: 10.1111/j.1477-2574.2011.00390.x. PMID: 22081925. PMCID: PMC3244629.

36. DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, et al. 2006; Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 244:931–937. discussion 937–939. DOI: 10.1097/01.sla.0000246856.03918.9a. PMID: 17122618. PMCID: PMC1856636.
37. Shia BC, Qin L, Lin KC, Fang CY, Tsai LL, Kao YW, et al. 2020; Age comorbidity scores as risk factors for 90-day mortality in patients with a pancreatic head adenocarcinoma receiving a pancreaticoduodenectomy: a national population-based study. Cancer Med. 9:562–574. DOI: 10.1002/cam4.2730. PMID: 31789464. PMCID: PMC6970054.

38. Eeson G, Chang N, McGahan CE, Khurshed F, Buczkowski AK, Scudamore CH, et al. 2012; Determination of factors predictive of outcome for patients undergoing a pancreaticoduodenectomy of pancreatic head ductal adenocarcinomas. HPB (Oxford). 14:310–316. DOI: 10.1111/j.1477-2574.2012.00448.x. PMID: 22487068. PMCID: PMC3384850.

39. Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R, et al. 2011; A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 254:702–707. discussion 707–708. DOI: 10.1097/SLA.0b013e31823598fb. PMID: 22042466.

40. Wiltberger G, Muhl B, Benzing C, Atanasov G, Hau HM, Horn M, et al. 2016; Preoperative risk stratification for major complications following pancreaticoduodenectomy: identification of high-risk patients. Int J Surg. 31:33–39. DOI: 10.1016/j.ijsu.2016.04.034. PMID: 27126297.

41. Scheufele F, Aichinger L, Jäger C, Demir IE, Schorn S, Demir E, et al. 2021; INR and not bilirubin levels predict postoperative morbidity in patients with malignant obstructive jaundice. Am J Surg. 222:976–982. DOI: 10.1016/j.amjsurg.2021.04.016. PMID: 34001332.

42. Pamecha V, Sadashiv Patil N, Kumar S, Rajendran V, Gupta S, Vasantrao Sasturkar S, et al. 2019; Upfront pancreaticoduodenectomy in severely jaundiced patients: is it safe? J Hepatobiliary Pancreat Sci. 26:524–533. DOI: 10.1002/jhbp.671. PMID: 31532900.

43. Wang S, Wang X, Li L, Dai H, Han J. 2013; Association of preoperative obstructive jaundice with postoperative infectious complications following pancreaticoduodenectomy. Hepatogastroenterology. 60:1274–1279.
44. Pavlidis ET, Pavlidis TE. 2018; Pathophysiological consequences of obstructive jaundice and perioperative management. Hepatobiliary Pancreat Dis Int. 17:17–21. DOI: 10.1016/j.hbpd.2018.01.008. PMID: 29428098.

45. Mantovani A, Allavena P, Sica A, Balkwill F. 2008; Cancer-related inflammation. Nature. 454:436–444. DOI: 10.1038/nature07205. PMID: 18650914.

46. Clark EJ, Connor S, Taylor MA, Madhavan KK, Garden OJ, Parks RW. 2007; Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB (Oxford). 9:456–460. DOI: 10.1080/13651820701774891. PMID: 18345294. PMCID: PMC2215360.

47. Arikan T, Sozuer EM, Topal U, Dal F, Bozkurt GK, Yilmaz AZ. 2020; The value and prognostic significance of neutrophil /lymphocyte ratio in predicting pancreatic fistula in patients undergoing pancreaticoduodenectomy for periampullary tumors. Ann Med Res. 27:1214–1220. DOI: 10.5455/annalsmedres.2019.12.792.

48. Mowbray NG, Griffith D, Hammoda M, Shingler G, Kambal A, Al-Sarireh B. 2018; A meta-analysis of the utility of the neutrophil-to-lymphocyte ratio in predicting survival after pancreatic cancer resection. HPB (Oxford). 20:379–384. DOI: 10.1016/j.hpb.2017.12.009. PMID: 29336893.

49. Ida M, Tachiiri Y, Sato M, Kawaguchi M. 2019; Neutrophil-to-lymphocyte ratio as indicator to severe complication after pancreaticoduodenectomy or distal pancreatectomy. Acta Anaesthesiol Scand. 63:739–744. DOI: 10.1111/aas.13341. PMID: 30874307.

50. Huang H, Wang C, Ji F, Han Z, Xu H, Cao M. 2021; Nomogram based on albumin and neutrophil-to-lymphocyte ratio for predicting postoperative complications after pancreaticoduodenectomy. Gland Surg. 10:877–891. DOI: 10.21037/gs-20-789. PMID: 33842233. PMCID: PMC8033066.

51. Shen Z, Xu Z, Wang W, Xu W, Zhou Y, Lu X, et al. 2021; A novel nomogram for predicting the risk of major complications after pancreaticoduodenectomy in patients with obstructive jaundice. Clin Chim Acta. 517:162–170. DOI: 10.1016/j.cca.2021.02.018. PMID: 33711328.

Fig. 1
Flow diagram showing the selection of the study cohort. PD, pancreatoduodenectomy; PDAC, pancreatic ductal adenocarcinoma; AA, ampullary adenocarcinoma; CC, cholangiocarcinoma.
Fig. 2
(A) Survival curves by histology. (B) Time-to-recurrence curves by histology. PDAC, pancreatic ductal adenocarcinoma; AA, ampullary adenocarcinoma; CC, cholangiocarcinoma.
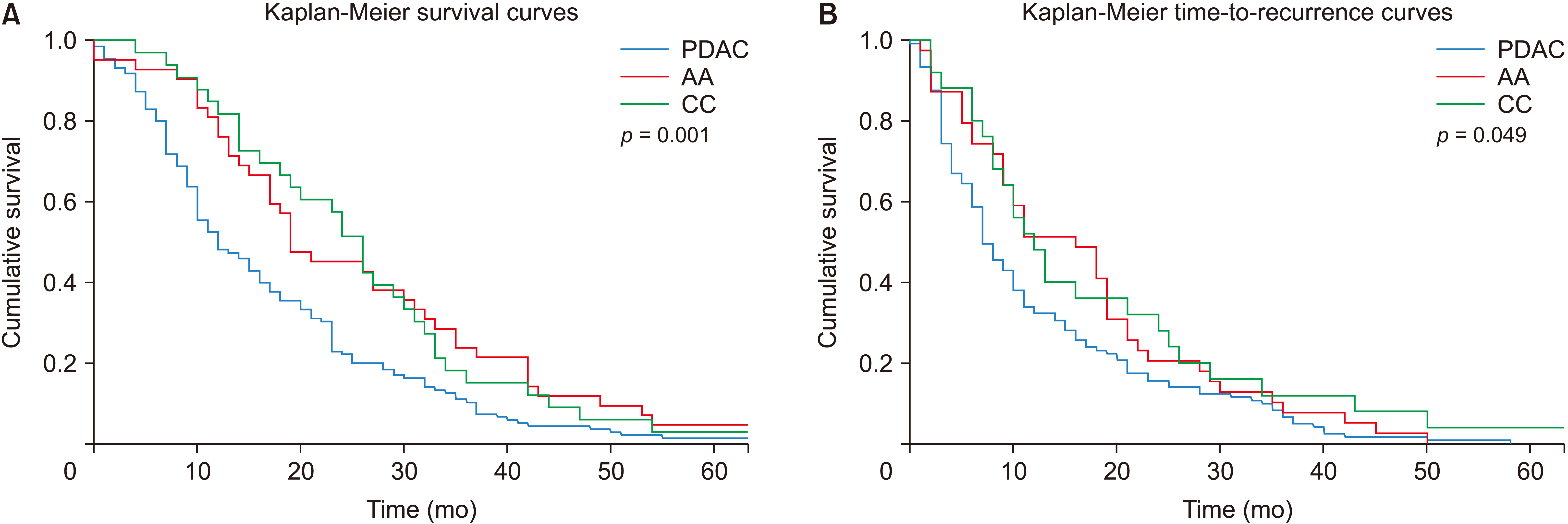
Table 1
Demographics of patients who underwent PD for histologically-confirmed PDAC, AA, or CC
| Variable | All patients (n = 271) | PDAC (n = 157) | AA (n = 70) | CC (n = 44) | p-value |
|---|---|---|---|---|---|
| Age (yr) | 66 (33–83) | 67 (41–82) | 66.5 (33–83) | 65 (42–83) | 0.871 |
| Body mass index (kg/m2) | 25.9 (16.4–53.4) | 25.1 (16.4–53.3) | 26.7 (19.2–41.6) | 25.9 (16.6–36.9) | 0.080 |
| Comorbidity | |||||
| Diabetes | 42 (15.5) | 32 (20.4) | 7 (10.0) | 2 (4.5) | 0.013* |
| Cardiovascular | 104 (38.4) | 64 (40.8) | 22 (31.4) | 18 (40.9) | 0.381 |
| Respiratory | 46 (17.0) | 24 (15.3) | 9 (12.9) | 13 (29.5) | 0.048* |
| Prior history of cancer | 28 (10.3) | 20 (12.7) | 4 (5.7) | 4 (9.1) | 0.263 |
| Pre-op treatment | |||||
| Biliary stent | 221 (81.5) | 124 (79.0) | 58 (82.9) | 39 (88.6) | 0.327 |
| Chemotherapy | 3 (1.1) | 3 (1.9) | 0 (0.0) | 0 (0.0) | - |
| Radiotherapy | 2 (0.8) | 2 (1.3) | 0 (0.0) | 0 (0.0) | - |
| Pre-op blood tests | |||||
| Bilirubin (µmol/L) | 29 (3–916) | 30 (3–916) | 26 (4–288) | 36.5 (6–277) | 0.353 |
| Albumin (g/L) | 40 (12–51) | 40 (21–51) | 40.5 (21–48) | 42 (22–49) | 0.363 |
| Neutrophils (×109/L) | 5.2 (1.6–29) | 5.1 (1.6–29) | 5.5 (2.6–20) | 4.9 (2.2–14.2) | 0.549 |
| Lymphocytes (×109/L) | 1.8 (0.2–7.1) | 1.8 (0.2–5.0) | 1.7 (0.6–7.1) | 1.9 (0.5–3.0) | 0.780 |
| NLR | 3.1 (0.5–28.4) | 3.2 (0.9–28.4) | 2.8 (0.5–22.8) | 3.2 (1.4–28.4) | 0.891 |
| ASA grade I/II |
177 (65.3) Unknown: 9 |
102 (66.7) Unknown: 4 |
50 (73.5) Unknown: 2 |
24 (58.5) Unknown: 3 |
0.266 |
| Type of pancreatic anastomosis | |||||
| Not performed | 1 (0.4) | 0 (0.0) | 1 (1.4) | 0 (0.0) | - |
| PG | 195 (72.0) | 121 (77.1) | 44 (62.9) | 30 (68.2) | 0.073 |
| PJ | 75 (27.7) | 36 (22.9) | 25 (35.7) | 14 (31.2) | 0.110 |
| Vascular resection performed | |||||
| Venous | 35 (12.9) | 32 (20.4) | 1 (1.4) | 2 (4.5) | < 0.001* |
| Arterial | 7 (2.6) | 6 (3.2) | 0 (0.0) | 1 (2.3) | 0.620 |
| Intra-operative blood transfusion received | 32 (11.8) | 22 (14.0) | 8 (11.4) | 2 (4.5) | 0.323 |
| Post-op destination | |||||
| Critical care | 197 (72.7) | 121 (77.1) | 50 (71.4) | 26 (59.1) | 0.059 |
| Surgical ward | 74 (27.3) | 36 (22.9) | 20 (28.6) | 18 (40.9) | 0.059 |
| Post-op nutritional support received | 81 (29.9) | 42 (26.8) | 22 (31.4) | 17 (38.6) | 0.300 |
| 30-day return to theatre | 15 (5.5) | 6 (3.8) | 6 (8.6) | 3 (6.8) | 0.324 |
| Median length of stay (day) | 11 (3–102) | 10 (3–69) | 11 (3–102) | 12 (5–50) | 0.842 |
| 30-day readmission | 18 (6.6) | 12 (7.6) | 4 (5.7) | 2 (4.5) | 0.732 |
| 90-day mortality | 9 (3.3) | 6 (3.8) | 3 (4.3) | 0 (0.0) | 0.869 |
| Tumour size (mm) | 30 (5–130) | 32 (12–130) | 24 (5–80) | 24.5 (10–50) | < 0.001* |
| Resection margin (R) status | |||||
| R0 | 101 (37.3) | 30 (19.1) | 53 (75.7) | 26 (59.1) | < 0.001* |
| R1 | 166 (61.3) | 123 (78.3) | 17 (24.3) | 18 (40.9) | < 0.001* |
| R2 | 4 (1.5) | 4 (2.5) | 0 (0.0) | 0 (0.0) | - |
| Number of resected nodes | 16 (1–38) | 16 (1–34) | 15 (2–33) | 16 (4–38) | 0.299 |
| Number of involved nodes | 2 (0–21) | 4 (0–21) | 1 (0–11) | 2 (0–12) | < 0.001* |
Table 2
Recorded complications
Table 3
Selected preoperative factors and their associations with morbidity (any complication), major morbidity (at least one complication Clavien-Dindo grade III or higher), 90-day mortality and five-year survival (comorbidity refers to a preoperative diagnosis of diabetes mellitus, cardiovascular disease or respiratory disease)
| Preoperative variable/factor | Morbidity (%) | p-value | Major morbidity (%) | p-value | 90-day mortality (%) | p-value | 5-year survival (%) | p-value |
|---|---|---|---|---|---|---|---|---|
| Age (yr) | ||||||||
| ≤ 66 | 69.1 | 0.698 | 17.3 | > 0.999 | 2.2 | 0.325 | 21.6 | 0.772 |
| > 66 | 66.7 | 17.4 | 4.5 | 24.2 | ||||
| BMI (kg/m2) | ||||||||
| ≤ 25.9 | 65.3 | 0.579 | 20.3 | 0.500 | 2.5 | 0.500 | 21.2 | 0.642 |
| > 25.9 | 69.5 | 16.1 | 5.1 | 24.6 | ||||
| Comorbidity | ||||||||
| Pre-op comorbidity | 70.7 | 0.297 | 13.3 | 0.055 | 2.7 | 0.519 | 21.3 | 0.662 |
| No pre-op comorbidity | 64.5 | 22.3 | 4.1 | 24.0 | ||||
| ASA grade | ||||||||
| I–II | 62.1 | 0.002* | 13.0 | 0.009* | 2.3 | 0.155 | 24.3 | 0.539 |
| III–IV | 81.2 | 27.1 | 5.9 | 18.8 | ||||
| Pre-op bilirubin (µmol/L) | ||||||||
| ≤ 29 | 61.6 | 0.027* | 11.6 | 0.016* | 2.9 | 0.746 | 26.8 | 0.109 |
| > 29 | 74.4 | 23.3 | 3.8 | 18.0 | ||||
| Pre-op albumin (g/L) | ||||||||
| ≤ 40 | 68.4 | 0.522 | 16.9 | 0.874 | 2.9 | 0.748 | 20.6 | 0.470 |
| > 40 | 64.4 | 17.8 | 3.7 | 24.4 | ||||
| NLR | ||||||||
| ≤ 3.1 | 61.8 | 0.037* | 11.2 | 0.006* | 2.2 | 0.334 | 25.7 | 0.245 |
| > 3.1 | 74.1 | 23.7 | 4.4 | 19.3 |
Table 4
Postoperative treatment, recurrence and survival statistics
| Postoperative treatment/long-term outcome | All patients (n = 271) | PDAC (n = 157) | AA (n = 70) | CC (n = 44) | p-value |
|---|---|---|---|---|---|
| Adjuvant chemotherapy received | < 0.001* | ||||
| Yes | 151 (57.2) | 101 (65.6) | 24 (35.3) | 26 (61.9) | |
| Unknown | 7 | 3 | 2 | 2 | |
| Completed planned course | 0.253 | ||||
| Yes | 113 (75.8) | 73 (73.0) | 17 (73.9) | 23 (88.5) | |
| Unknown | 2 | 1 | 1 | ||
| 5-year cancer recurrence | 185 (68.3) | 121 (77.1) | 39 (55.7) | 25 (56.8) | 0.001* |
| Median time to recurrence in months (range) | 9 (0–58) | 7 (0–58) | 16 (1–50) | 11.5 (2–43) | 0.017* |
| Palliative chemotherapy receiveda) | 0.414 | ||||
| Yes | 58 (31.4) | 34 (28.0) | 15 (38.5) | 9 (36.0) | |
| Unknown | 7 | 3 | 2 | 2 | |
| 5-year survival | 61 (22.5) | 22 (14.0) | 28 (40.0) | 11 (25.0) | 0.001* |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download