Abstract
Backgrounds/Aims
Data regarding outcomes of endoscopic retrograde cholangiography (ERC) in liver transplant (LT) recipients with biliary-enteric (BE) anastomosis are limited. We report outcomes of ERC and percutaneous transhepatic biliary drainage (PTBD) as first-line therapies in LT recipients with BE anastomosis.
Methods
All LT recipients with Roux-BE anastomosis from 2001 to 2020 were divided into ERC and PTBD subgroups. Technical success was defined as the ability to cannulate the bile duct. Clinical success was defined as the ability to perform cholangiography and therapeutic interventions.
Results
A total of 36 LT recipients (25 males, age 53.5 ± 13 years) with Roux-BE anastomosis who underwent biliary intervention were identified. The most common indications for a BE anastomosis were primary sclerosing cholangitis (n = 14) and duct size mismatch (n = 10). Among the 29 patients who initially underwent ERC, technical success and clinical success were achieved in 24 (82.8%) and 22 (75.9%) patients, respectively. The initial endoscope used for the ERC was a single balloon enteroscope in 16 patients, a double balloon enteroscope in 7 patients, a pediatric colonoscope in 5 patients, and a conventional reusable duodenoscope in 1 patient. Among the 7 patients who underwent PTBD as the initial therapy, six (85.7%) achieved technical and clinical success (p = 0.57).
Anastomotic and non-anastomotic biliary strictures are common causes of morbidity after orthotopic liver transplantation (LT). Endoscopic retrograde cholangiography (ERC) is widely considered the preferred modality for management of post-LT strictures [1,2], especially in patients with duct-to-duct anastomosis. However, management of biliary strictures in LT recipients with a Roux-en-Y biliary-enteric (BE) anastomosis has not been explored in detail.
Traditionally, percutaneous transhepatic biliary drainage (PTBD) with or without cholangioplasty has been the first-line therapy for biliary strictures in LT recipients with BE anastomosis whereas ERC has been advocated as the first line therapy for those with a conventional choledocho-choledochal anastomosis [3,4]. Data suggest that PTBD with balloon cholangioplasty is an effective strategy for the management of post-LT strictures [5,6]. Indeed, PTBD is considered the initial preference by some institutions for all LT recipients irrespective of foregut anatomy, especially living donor recipients [7,8].
Although a few investigators have reported their experience with ERC in LT recipients with a BE anastomosis [9,10], the literature remains sparse. It is often restricted to patients undergoing ERC only. In this study, we report our experience of ERC and PTBD as the primary therapy in LT recipients with a Roux-en-Y BE anastomosis. We hypothesized that ERC and PTBD would have comparable efficacy as primary therapy in this patient population.
This study was approved by the institutional review board at Mayo Clinic, Arizona (15-008386). In this retrospective study, all patients who underwent LT at the Mayo Clinic in Arizona from January 2001 to December 2020 were selected from a prospectively maintained database. Adult LT recipients who received a Roux-en-Y BE anastomosis (choledocho-jejunostomy or hepaticojejunostomy) and required biliary interventions were included in this study. Pediatric allograft LT recipients, patients with unaltered foregut anatomy, and patients without any post-LT biliary pathology were excluded. Patients who underwent Roux-en-Y BE anastomosis for reasons unrelated to LT such pancreaticoduodenectomy, palliative biliary bypass, or gastric bypass for weight loss were also excluded.
Patient demographics, indications for LT, preoperative characteristics, and peri-operative characteristics were recorded. Biochemical profiles of LT recipients were assessed using electronic medical records. The length of the Roux limb was calculated from the ligament of Treitz and recorded from the operative report. Patients were subdivided into those who underwent ERC and those who underwent PTBD based on the initial intervention performed. The choice of PTBD or ERC was based on the discretion of the treating physician.
Patients who initially underwent ERC for management of biliary pathology after LT were included in the endoscopy group. ERC procedures performed prior to LT were not considered for the analysis. The endoscopy database and electronic medical record were used to ascertain the presence, location, and type of biliary strictures. The total time for endoscopy was calculated from the time of intubation with endoscope to its final removal from the patient. Fluoroscopy time was recorded from the radiology report. In patients who had more than one ERC, only the first procedure was included in the analysis of outcomes. All LT recipients undergoing ERC were considered to have a high risk for infections. They received periprocedural antibiotics per institutional protocol and clinical guidelines [11,12].
Technical success was defined as the ability to reach the BE anastomosis. Clinical success was defined as the ability to perform cholangiography and therapeutic interventions, if needed. Peri-procedural adverse events were recorded and classified based on previously published criteria [13].
Patients who initially underwent PTBD as the initial intervention after LT were included in the PTBD group. Procedure details and demographics were obtained from medical records. Technical success was defined as the ability to access bile ducts. Clinical success was defined as the ability to perform cholangiography and therapeutic interventions such as placement of a drain or catheter as described previously [14].
Liver allografts were procured locally and regionally. All grafts were procured by Mayo Clinic Transplant surgery team per our standard policy. This policy entails that the recipient is taken to the operating room immediately after the transplant procurement team confirms the suitability of the procured graft. This policy coupled with appropriate selection of recipient with minimal complexity and lower Model for End-stage Liver Disease score can keep the cold ischemia time relatively short [1]. Rapid retrieval techniques were employed for all organ procurements followed by a back table dissection. None of the patients had a normothermic perfusion.
Anastomoses were created using standard techniques based on operative anatomy. The biliary portion of the anastomosis was created using an end-to-side technique with an absorbable monofilament suture (typically 5-0 or 6-0 polydioxanone) in a continuous or running fashion per surgeon discretion. The jejunojejunostomy was created using the standard two-layer handsewn technique. Typically, an interrupted silk suture was used for the outer layer and an absorbable suture was used for the inner layer.
All patients received standard calcineurin inhibitor-based immunosuppression in combination with mycophenolate mofetil and prednisone. Both mycophenolate mofetil and prednisone were tapered off within 4 months.
Continuous variables were compared using unpaired two-tailed Student’s t-test and presented as mean ± standard deviation or median (range). Categorical variables were compared using χ2 or Fisher’s exact test and reported as counts and percentages. A p-value < 0.05 was considered statistically significant.
At our center, 1,663 liver transplants were performed, including 96 who underwent a Roux-en-Y hepaticojejunostomy. A total of 36 LT recipients (25 males and 11 females) with a mean age of 53.5 years at LT who had a Roux anatomy required biliary interventions (Table 1, Fig. 1). Twenty-nine patients had a retro-colic anastomosis. Three patients had an ante-colic anastomosis. The type of anastomosis was not known for the remaining four patients. Five patients had undergone at least one revision of Roux anastomosis prior to biliary intervention. Overall, the most common indication for LT was primary sclerosing cholangitis mediated chronic liver disease. Biliary interventions were most often performed for management of presumed post-LT biliary strictures based on elevated liver associated enzymes or radiographic evidence of biliary stricture.
A total of 29 LT recipients with Roux-en-Y BE anastomosis underwent ERC as the initial intervention. The median time from LT to ERC was 131 days (interquartile range: 65–503 days). The initial endoscope used for the ERC was a single balloon enteroscope in 16 patients, a double balloon enteroscope in 7 patients, a pediatric colonoscope in 5 patients, and a conventional reusable duodenoscope in 1 patient. The duration of fluoroscopy was 10.7 ± 11.25 minutes (mean ± standard deviation). The total endoscopy time was 86.8 ± 47.6 minutes.
Of the 29 patients who underwent ERC, technical success was achieved in 24 (82.8%) patients and clinical success was achieved in 22 (75.9%) patients. Due to the lack of clinical success with ERC, the remaining 7 patients were referred for PTBD. In five patients, the endoscope could not be navigated to the BE anastomosis. These five patients included four patients in whom a balloon enteroscope was used and one patient in whom a duodenoscope was used. In another patient, the choledocho-jejunal anastomosis could be reached. However, the bile duct could not be cannulated. Finally, in one patient, although cannulation of the bile duct was successful, the desired intrahepatic duct could not be cannulated.
Among the 7 patients who failed ERC, 5 patients successfully underwent PTBD whereas 1 patient was referred for surgical intervention after being diagnosed with an afferent limb obstruction. Another patient with elevated liver enzymes failed ERC and PTBD. After a multi-disciplinary review, the patient was closely followed without therapeutic intervention and was noted to be doing well clinically. One patient who underwent a successful PTBD after failed ERC subsequently underwent ERC with internalization of PTBD drain.
Anastomotic strictures, intrahepatic strictures, and choledocholithiasis were found in 15, 3, and 2 patients, respectively. Endoscopic dilation with stenting, stenting alone, and balloon sweeps were performed in 10, 6, and 2 patients, respectively. Plastic stents of 7 French diameter were used most frequently. In 6 patients with elevated liver enzymes and clinical suspicion for post-LT strictures, no significant pathology was seen on cholangiography (Fig. 2). There were no peri-procedural complications.
Repeat ERC procedures (median number: 1; range, 0–15) were required in 21 patients, including one patient who required 15 ERC procedures for serial stenting of post-LT biliary strictures. There were no serious adverse events.
A total of 7 LT recipients underwent PTBD as the initial therapy for biliary intervention (Table 1). The time from LT to PTBD was 741.9 ± 833.6 days (median: 180 days). PTBD was technically and clinically successful in 6 LT recipients. Technical and clinical success rates were both 85.7% with PTBD. They were 82.7% and 75.8%, respectively, with ERC (p = 0.85 and p = 0.57, respectively). PTBD failed in one patient who was then referred for an ERC. One patient who initially underwent PTBD for cholangitis secondary to a biliary stricture subsequently underwent ERC for exchange of biliary drain with an internal stent. Four of the six patients who underwent PTBD successfully underwent repeated procedures (median: 1 repeat PTBD). There were no serious adverse events. Clinical and technical success rates were comparable between ERC and PTBD subgroups (p = 0.57 and p = 0.85, respectively).
Biliary stricture are the most common adverse outcomes of LT. They are associated with allograft rejection, allograft failure, infections, readmission to the hospital, and death [15]. These strictures vary in severity, etiology, and location [16]. The clinical impact of post-transplant biliary strictures can vary from mild asymptomatic elevation in liver enzymes or abnormal cross-sectional imaging to cholangitis and septic shock [17,18].
Typically, post-LT strictures are effectively managed with ERC [1,19]. However, the management is uniquely challenging in patients with a Roux-en-Y BE anastomosis. An altered foregut anatomy typically precludes the use of a side-viewing duodenoscope. It often requires the use of colonoscopes, enteroscopes, and specialized accessories [20,21]. This has led to a preferential utilization of PTBD as the primary modality of biliary intervention in these patients [4]. In one of the early studies on post-LT biliary complications published in 1994, Kuo et al. [3] reported their preference of ERC in patients with duct-to-duct anastomosis and PTBD in patients with a choledocho-jejunostomy. Our study noted a 75.8% success rate with primary endoscopic approach, suggesting that ERC might be equivalent to PTBD as the initial strategy in the management of post-LT strictures in patients with a BE anastomosis.
Duct-to-duct reconstruction remains the most frequently performed anastomosis. One systematic review reported that in 73% institutions, 92% of all deceased donor and 70% of living donor transplants used a duct-to-duct reconstruction [7]. A Roux-en-Y BE anastomosis is often required when the extrahepatic bile duct cannot be used for creating choledocho-choledochal anastomosis. This could occur due to intrinsic diseases of bile ducts (such as biliary atresia and primary sclerosing cholangitis), duct size discrepancy between donor and recipient ducts, or re-transplantation [7,22,23].
The most common indication for a Roux-en-Y BE anastomosis in our study was primary sclerosing cholangitis. Published literature suggests that a choledocho-choledochal anastomosis is associated with higher incidence of strictures and worse allograft survival compared with a Roux-en-Y anastomosis [24]. Based on these data published by Welsh and Wigmore [24], the American Association for the Study of Liver Diseases practice guideline states that a Roux-en-Y choledocho-jejunostomy is the preferred biliary anastomosis in primary sclerosing cholangitis [25].
Data on ERC outcomes in LT recipients with BE anastomosis for LT are limited, although there are some previous studies on patients with Whipple operation or a Roux-en-Y gastric bypass [26]. Data from endoscopic interventions used in Roux-en-Y gastric bypass patients cannot be extrapolated to LT recipients with BE anastomosis. The length of the bypassed foregut which must be traversed by the endoscope is much longer in patients with a Roux-en-Y gastric bypass than in LT recipients with BE anastomosis. Indeed, our study demonstrated that the mean length of the Roux (or alimentary) limb was 41.4 cm. In comparison, the Society of American Gastrointestinal and Endoscopic Surgeons guideline suggests that the length of the Roux limb is typically 150 cm in patients with a standard gastric bypass [27]. Thus, patients with a gastric bypass typically require a device/balloon assisted enteroscope, lumen-apposing metal stents, or laparoscopic assistance to access the biliary system whereas a device assisted enteroscope or a colonoscope can often suffice in LT recipients with BE anastomosis [21].
This study supports results of other studies on the role of ERC in patients with BE anastomosis from a variety of surgical procedures. Hammad et al. [28] from the University of Colorado reported their experience of 71 patients, of which only 28 were LT recipients. They reported comparable rates of technical success with ERC (76%) and PTBD (77%). In our study population of LT recipients with BE anastomosis, a similar success rate with ERC was also found. Chahal et al. [10] also reported a comparable success rate of 71% with endoscopic therapy, although they did not report outcomes of patients undergoing PTBD.
Interestingly, Hammad et al. [28], Chahal et al. [10], and the present study showed comparable outcomes despite utilizing different endoscopes. In the study by Hammad et al. [28], a colonoscope was used in patients with a post-Whipple anatomy whereas a rotational or balloon-overtube assisted enteroscope was used in most other patients, including LT recipients. In contrast, we did not utilize a spiral enteroscope. Instead, we used a balloon-assisted enteroscope more frequently. Chahal et al. [10] in their case series comprising of 31 patients used variable stiffness colonoscopes for most procedures without using an enteroscope at all. In a retrospective analysis comparing success rates of different endoscopes in this patient population, Azeem et al. [9] have reported a higher success rate with a single balloon enteroscope than with a variable stiffness adult or pediatric colonoscope.
Our results suggest that ERC and PTBD might be complementary procedures and that patients often require both techniques to achieve sustained clinical success. In our study, one patient who initially underwent a successful PTBD for management of a post-LT biliary stricture was subsequently managed by an endoscopic therapy. Similarly, two patients who initially underwent a successful ERC were subsequently managed by PTBD. Common causes of inability to reach BE anastomosis included long length of the Roux limb and looping in the stomach, or tight angulation at the entero-enteric Roux anastomosis. Similar causes of failure have been reported by others [10].
In patients who undergo PTBD, consideration could be given for a follow-up ERCP for internalization of the drain if needed. Notably, while PTBD and ERCP have comparable rates of success, PTBD might not be a favored modality due to the presence of external drains/catheters. Data from Lee et al. [29] have demonstrated that external drains in liver transplant recipients are associated with a higher risk of adverse events including leakage, retraction, patient discomfort, risk of dislodgement, and need for replacement. A recent multivariate analysis from a nationwide study among hospitalized liver transplant recipients has also reported that PTBD is associated with higher adjusted odds of failure of liver allograft, length of hospitalization, disposition to nursing home, and overall cost [15].
Based on our clinical experience and results of this study, we suggest a multi-disciplinary discussion in the management of patients with post-LT biliary strictures and a BE anastomosis. We also recommend a review of the operative report to identify the length of the roux limb, which can possibly guide the choice of the endoscope to be used for the procedure. Azeem et al. [9] have reported that intraoperative tattooing is useful for subsequent identification of the afferent limb. In our clinical practice, we often place a submucosal tattoo to mark the biliary limb of the jejunum to facilitate subsequent identification. In selected patients with intrahepatic biliary dilation, endoscopic ultrasound guided biliary access can also be considered [30]. Prior to performing endoscopic interventions, appropriate devices and tools can be selected based on the American Society for Gastrointestinal Endoscopy’s recommendations [21].
While the present study did not evaluate correlation between the roux limb length and outcomes by the type of endoscope, a device-assisted enteroscope could offer potential advantages in situations with redundant or long limbs. Additionally, adequate time should be allotted for these technically complex procedures. We found a mean procedure duration of 86.8 minutes whereas Hammad et al. [28] reported a mean procedure duration of 110 minutes and Chahal et al. [10] reported a median procedure duration of 43 minutes.
Our study is limited by its single-center, retrospective study design and relatively small sample size of patients with post-LT BE strictures. However, this is a unique population. Despite these limitations, this study is among the largest studies to date to examine the role of primary ERC and PTBD in a homogenous sample of LT recipients with a BE anastomosis rather than combining this group with post-Whipple and other procedures. The post liver transplant patient population is quite different from other post-surgical patients due to various clinical factors such as immunosuppression. Our study did not have patients who underwent a choledocho-duodenostomy anastomosis as this was relatively uncommon at our institution. However, ERC outcomes in patients with this anastomosis should be evaluated in future studies.
This study suggests that ERC and PTBD might be complementary techniques with comparable effectiveness as primary biliary interventions in this patient population. If expertise is available, ERC can be considered a first-line therapeutic intervention in LT recipients with a BE anastomosis as in patients with duct-to-duct anastomosis. Future multicenter studies are needed to evaluate patient and procedural variables that impact clinical and technical success rates of ERC in this population.
REFERENCES
1. Kohli DR, Harrison ME, Mujahed T, Fukami N, Faigel DO, Pannala R, et al. 2020; Outcomes of endoscopic therapy in donation after cardiac death liver transplant biliary strictures. HPB (Oxford). 22:979–986. DOI: 10.1016/j.hpb.2019.10.018. PMID: 31676256.

2. Chathadi KV, Chandrasekhara V, Acosta RD, Decker GA, Early DS, et al. ASGE Standards of Practice Committee. 2015; The role of ERCP in benign diseases of the biliary tract. Gastrointest Endosc. 81:795–803. DOI: 10.1016/j.gie.2014.11.019. PMID: 25665931.

3. Kuo PC, Lewis WD, Stokes K, Pleskow D, Simpson MA, Jenkins RL. 1994; A comparison of operation, endoscopic retrograde cholangiopancreatography, and percutaneous transhepatic cholangiography in biliary complications after hepatic transplantation. J Am Coll Surg. 179:177–181.
4. Ostroff JW. 2010; Management of biliary complications in the liver transplant patient. Gastroenterol Hepatol (N Y). 6:264–272.
5. Sung RS, Campbell DA Jr, Rudich SM, Punch JD, Shieck VL, Armstrong JM, et al. 2004; Long-term follow-up of percutaneous transhepatic balloon cholangioplasty in the management of biliary strictures after liver transplantation. Transplantation. 77:110–115. DOI: 10.1097/01.TP.0000101518.19849.C8. PMID: 14724444.

6. Mukund A, Choudhury A, Das S, Pamecha V, Sarin SK. 2020; Salvage PTBD in post living donor liver transplant patients with biliary complications-a single centre retrospective study. Br J Radiol. 93:20191046. DOI: 10.1259/bjr.20191046. PMID: 31971831. PMCID: PMC7362925.

7. Akamatsu N, Sugawara Y, Hashimoto D. 2011; Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 24:379–392. DOI: 10.1111/j.1432-2277.2010.01202.x. PMID: 21143651.

8. Piardi T, Greget M, Audet M, Calandra G, Gheza F, Ellero B, et al. 2011; Biliary strictures after liver transplantation: is percutaneous treatment indicated? Ann Transplant. 16:5–13. DOI: 10.12659/AOT.881858. PMID: 21716179.

9. Azeem N, Tabibian JH, Baron TH, Orhurhu V, Rosen CB, Petersen BT, et al. 2013; Use of a single-balloon enteroscope compared with variable-stiffness colonoscopes for endoscopic retrograde cholangiography in liver transplant patients with Roux-en-Y biliary anastomosis. Gastrointest Endosc. 77:568–577. DOI: 10.1016/j.gie.2012.11.031. PMID: 23369652.

10. Chahal P, Baron TH, Poterucha JJ, Rosen CB. 2007; Endoscopic retrograde cholangiography in post-orthotopic liver transplant population with Roux-en-Y biliary reconstruction. Liver Transpl. 13:1168–1173. DOI: 10.1002/lt.21198. PMID: 17663414.

11. Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, et al. ASGE Standards of Practice Committee. 2015; Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 81:81–89. DOI: 10.1016/j.gie.2014.08.008. PMID: 25442089.

12. Kohli DR, Shah TU, BouHaidar DS, Vachhani R, Siddiqui MS. 2018; Significant infections in liver transplant recipients undergoing endoscopic retrograde cholangiography are few and unaffected by prophylactic antibiotics. Dig Liver Dis. 50:1220–1224. DOI: 10.1016/j.dld.2018.05.014. PMID: 29907534.

13. Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, et al. 2010; A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 71:446–454. DOI: 10.1016/j.gie.2009.10.027. PMID: 20189503.

14. Morita S, Kitanosono T, Lee D, Syed L, Butani D, Holland G, et al. 2012; Comparison of technical success and complications of percutaneous transhepatic cholangiography and biliary drainage between patients with and without transplanted liver. AJR Am J Roentgenol. 199:1149–1152. DOI: 10.2214/AJR.11.8281. PMID: 23096192.

15. Kohli DR, Desai MV, Kennedy KF, Pandya P, Sharma P. 2021; Patients with post-transplant biliary strictures have significantly higher rates of liver transplant failure and rejection: a nationwide inpatient analysis. J Gastroenterol Hepatol. 36:2008–2014. DOI: 10.1111/jgh.15388. PMID: 33373488.

16. Kohli DR, Pannala R, Crowell MD, Fukami N, Faigel DO, Aqel BA, et al. 2021; Interobserver agreement for classifying post-liver transplant biliary strictures in donation after circulatory death donors. Dig Dis Sci. 66:231–237. DOI: 10.1007/s10620-020-06169-7. PMID: 32124198.

17. Fasullo M, Kandakatla P, Amerinasab R, Kohli DR, Shah T, Patel S, et al. 2022; Early laboratory values after liver transplantation are associated with anastomotic biliary strictures. Ann Hepatobiliary Pancreat Surg. 26:76–83. DOI: 10.14701/ahbps.21-103. PMID: 35013006. PMCID: PMC8901979.

18. Kohli DR, Vachhani R, Shah TU, BouHaidar DS, Siddiqui MS. 2017; Diagnostic accuracy of laboratory tests and diagnostic imaging in detecting biliary strictures after liver transplantation. Dig Dis Sci. 62:1327–1333. DOI: 10.1007/s10620-017-4515-0. PMID: 28265825.

19. Kohli DR, Harrison ME, Adike AO, El Kurdi B, Fukami N, Faigel DO, et al. 2019; Predictors of biliary strictures after liver transplantation among recipients of DCD (donation after cardiac death) grafts. Dig Dis Sci. 64:2024–2030. DOI: 10.1007/s10620-018-5438-0. PMID: 30604376.

20. Kohli DR, Baillie J. Chandrasekhara V, Elmunzer BJ, Khashab MA, Muthusamy VR, editors. 2019. How endoscopes work. Clinical gastrointestinal endoscopy. 3rd ed. Elsevier;Philadelphia: p. 24–31.

21. Enestvedt BK, Kothari S, Pannala R, Yang J, Fujii-Lau LL, et al. ASGE Technology Committee. 2016; Devices and techniques for ERCP in the surgically altered GI tract. Gastrointest Endosc. 83:1061–1075. DOI: 10.1016/j.gie.2016.03.018. PMID: 27103361.

22. Wojcicki M, Milkiewicz P, Silva M. 2008; Biliary tract complications after liver transplantation: a review. Dig Surg. 25:245–257. DOI: 10.1159/000144653. PMID: 18628624.

23. Vallera RA, Cotton PB, Clavien PA. 1995; Biliary reconstruction for liver transplantation and management of biliary complications: overview and survey of current practices in the United States. Liver Transpl Surg. 1:143–152. DOI: 10.1002/lt.500010302. PMID: 9346556.

24. Welsh FK, Wigmore SJ. 2004; Roux-en-Y choledochojejunostomy is the method of choice for biliary reconstruction in liver transplantation for primary sclerosing cholangitis. Transplantation. 77:602–604. DOI: 10.1097/01.TP.0000113807.74942.D2. PMID: 15084943.

25. Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. 2010; Diagnosis and management of primary sclerosing cholangitis. Hepatology. 51:660–678. DOI: 10.1002/hep.23294. PMID: 20101749.

26. Kadaba RS, Bowers KA, Khorsandi S, Hutchins RR, Abraham AT, Sarker SJ, et al. 2017; Complications of biliary-enteric anastomoses. Ann R Coll Surg Engl. 99:210–215. DOI: 10.1308/rcsann.2016.0293. PMID: 27659373. PMCID: PMC5450270.

27. SAGES Guidelines Committee. 2008; SAGES guideline for clinical application of laparoscopic bariatric surgery. Surg Endosc. 22:2281–2300. DOI: 10.1007/s00464-008-9913-0. PMID: 18791862.
28. Hammad H, Brauer BC, Smolkin M, Ryu R, Obuch J, Shah RJ. 2019; Treating biliary-enteric anastomotic strictures with enteroscopy-ERCP requires fewer procedures than percutaneous transhepatic biliary drains. Dig Dis Sci. 64:2638–2644. DOI: 10.1007/s10620-019-05670-y. PMID: 31129875.

29. Lee SH, Ryu JK, Woo SM, Park JK, Yoo JW, Kim YT, et al. 2008; Optimal interventional treatment and long-term outcomes for biliary stricture after liver transplantation. Clin Transplant. 22:484–493. DOI: 10.1111/j.1399-0012.2008.00813.x. PMID: 18318735.

30. Jovani M, Ichkhanian Y, Vosoughi K, Khashab MA. 2019; EUS-guided biliary drainage for postsurgical anatomy. Endosc Ultrasound. 8(Suppl 1):S57–S66. DOI: 10.4103/eus.eus_53_19. PMID: 31897381. PMCID: PMC6896432.

Fig. 1
Liver transplant recipients with Roux-en-Y BE anastomosis. BE, biliary enteric; ERC, endoscopic retrograde cholangiography; PTBD, percutaneous transhepatic biliary drainage.
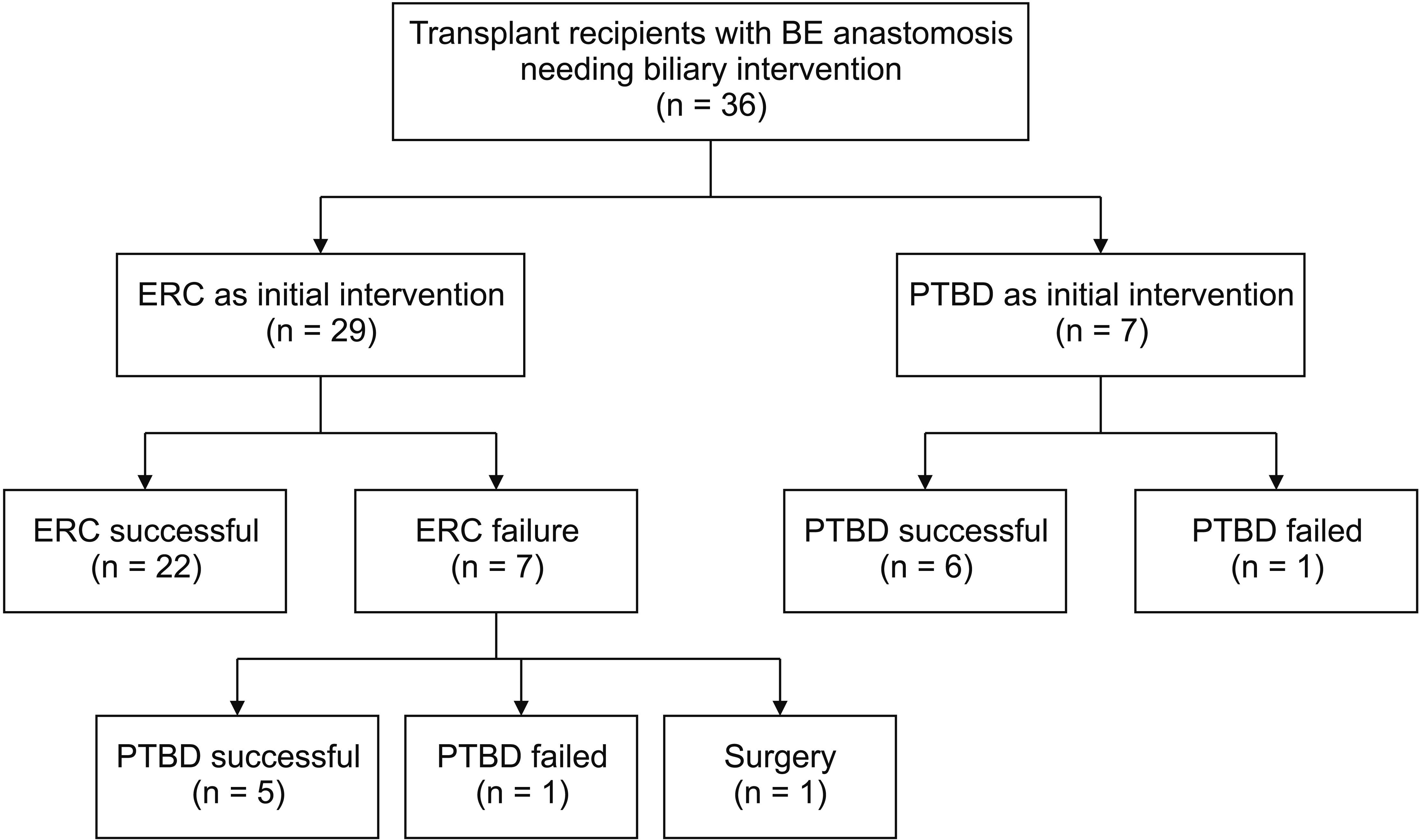
Fig. 2
Endoscopic retrograde cholangiography in a liver transplant recipient with biliary-enteric anastomosis.
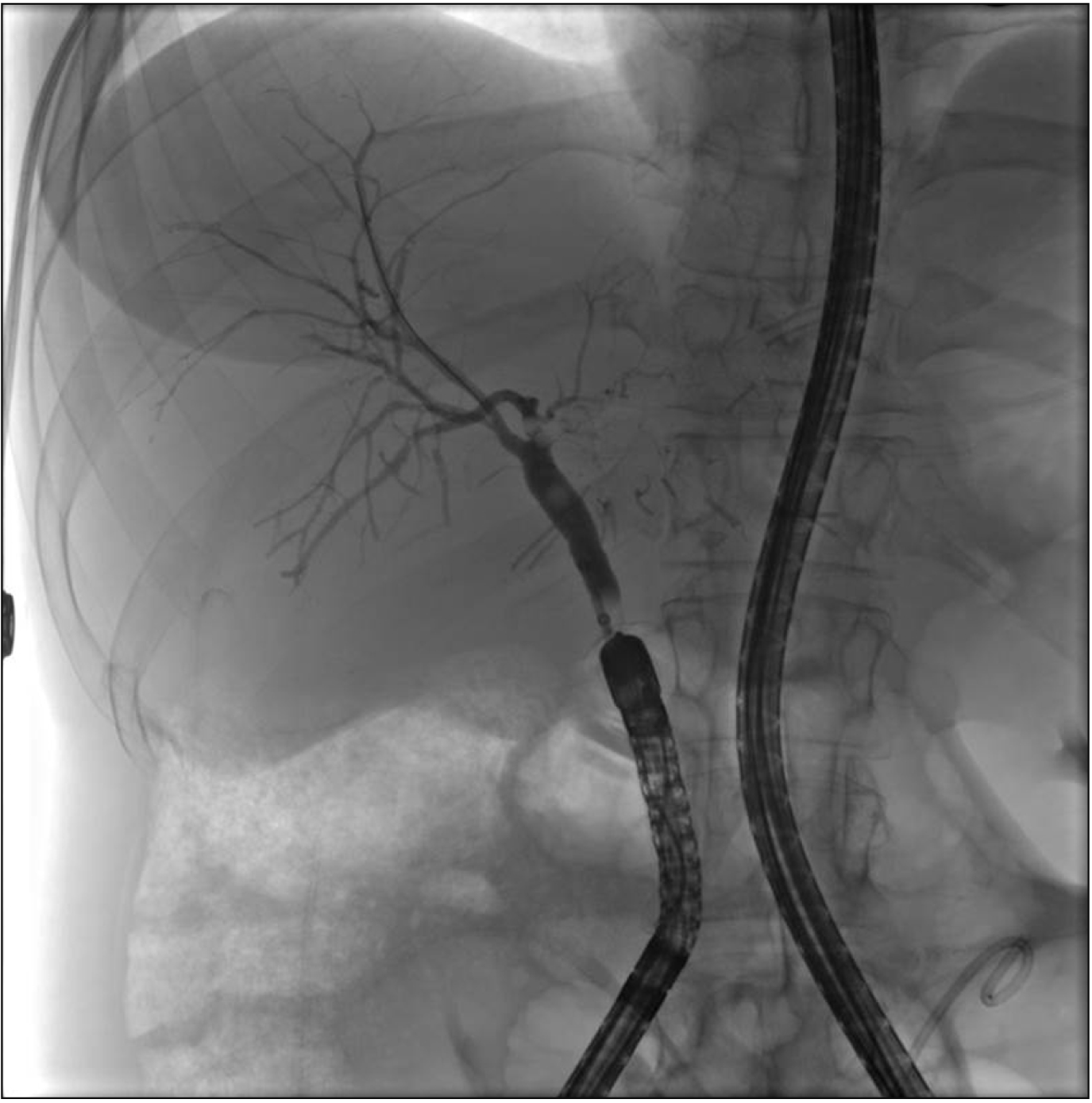
Table 1
Demographics of cohort




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download