Abstract
Backgrounds/Aims
Locally advanced gallbladder cancer (GBC) is associated with survival limited to a few months. Extended resections (ER) are occasionally performed in this group and outcomes remain inconclusive. This study assessed outcomes after ER for locally advanced GBC.
Methods
Patients who underwent ER for GBC between 2011 and 2020 were reviewed. ER was defined as a major hepatectomy alone (n = 9), a pancreaticoduodenectomy (PD) with or without minor hepatectomy (n = 3), a major hepatectomy with PD (HPD) (n = 3) or vascular resection and reconstruction (n = 4). We assessed 30-day morbidity, mortality, and 2-year overall survival (OS).
Results
Among 19 patients, negative margins were achieved in 14 (73.6%). The 30-day mortality was 1/9 (11.1%) for a major hepatectomy, 0/3 (0%) for a minor HPD, 2/3 (66.7%) for a major HPD, and 1/4 (25.0%) for vascular resection. All short term survivors (< 6 months) (n=8) had preoperative jaundice and 6/8 (75.0%) underwent a major HPD or vascular resection. There were five (26.3%) long term survivors. The median OS in patients with and without preoperative jaundice was 4.1 months (0.7–11.1 months) and 13.7 months (12–30.4 months), respectively (p = 0.009) (2-year OS = 7% vs. 75%; p = 0.008). The median OS in patients who underwent a major hepatectomy alone or a minor HPD was 11.3 months (6.8–17.3 months) versus 1.4 months (0.3–4.1 months) (p = 0.02) in patients who underwent major HPD or vascular resection (2 year OS = 33% vs. not reached) (p = 0.010) respectively.
Gallbladder cancer (GBC) is a rare tumor with a worldwide incidence rate of 2 per 100,000 [1,2]. However, considerable geographic variation is noted and some countries in South America and Asia have incidence rates as high as 13.8–21.5 per 100,000 [3]. Among biliary cancers, it is the most frequent type, with the shortest survival from the time of diagnosis [4]. GBC has an aggressive behavior with early invasion of adjacent organs, regional lymph nodes, and metastasis. As a result, most patients are diagnosed at an advanced stage, when curative treatment is not possible [5].
While surgical resection is the only curative treatment, approximately 10%–20% of patients are amenable to resection at presentation [6,7]. For complete tumor removal of T3–T4 GBC, extended resections (ER) such as a major hepatectomy, pancreaticoduodenectomy (PD), a combined hepatopancreaticoduodenectomy or vascular resection and reconstruction are an option. These resections are generally associated with high morbidity (50%), mortality (20%), and uncertain survival benefit, and are only attempted occasionally [7-9]. Patient selection remains a challenge and long term survival is possible in a few selected patients.
Without treatment, advanced GBC has an extremely poor prognosis and survival is limited to a few months [10]. There is a lack of data on survival after ER for GBC and results remain conflicting [7]. The need to improve patient selection and the role of ER in advanced GBC remains less well defined. This study aimed to assess the results of ERs for patients with advanced GBC at a single center and looked at factors associated with short and long term survival.
We reviewed patients who underwent curative intent surgery for GBC between 2011 and 2020 at our center. A total of 36 patients were identified. Patients who underwent ER for GBC were included (n = 19) in this study. We defined ER as a major hepatectomy (> 3 liver segments), a PD, or both with or without additional organ resection. In addition, patients requiring arterial or venous resection and reconstruction were also included [7]. The remaining 17 patients underwent wedge resection of the liver bed with portal lymphadenectomy or ≤ 3 segment liver resection.
All patients with suspected cancer of biliary origin, based on their clinical history and laboratory investigations, were staged with a computed tomography (CT) chest and dynamic CT scan of the liver. After discussion in the multi-disciplinary team meeting, further management was planned accordingly. Data on demographics, preoperative jaundice and biliary drainage, CA19-9, tumor related factors, treatment details, morbidity, recurrence and mortality was retrieved from patient files and electronic records. Tumor staging was performed according to the American Joint Committee on Cancer (AJCC) staging [11]. Broadly speaking, ER was considered for T3-T4 GBC in patients with a good performance status. The decision to perform a major hepatectomy was complex and based on the extent of hepatic arterial, portal venous, and biliary involvement. Similarly, PD was performed when margins from the distal bile duct were positive upon an intra operative frozen section, and PD was required to achieve negative margins. Gemcitabine monotherapy, or in combination with oxaliplatin, was used in the adjuvant setting. Patients with positive resection margins were also offered radiation therapy postoperative complications were graded based on Clavien–Dindo classification and grade 3A and above complications were recorded [12]. In-hospital mortality was defined as death during index hospital admission after surgical resection. Overall survival was calculated from the date of surgery until the last follow up, or death from any cause. Short term survival was defined as survival upto six months and long term survival was defined as survival > 12 months.
Categorical variables were presented as numbers (%). Continuous variables were presented as median (interquartile range) and the Mann–Whitney U test was used to determine significance. Survival was reported using Kaplan Meier curves and a log-rank test was used to determine significance. p-value < 0.05 was considered statistically significant. All statistical analysis was performed using SPSS Statistics for Windows, version 20 (IBM Corp., Armonk, NY, USA). The hospital ethics committee approved the study (IRB# 006-22).
Among 19 patients who underwent ER for GBC, there were 12 males (63.2%) and 7 females (36.8%), with a median age of 58 years (49–72 years) at the time of surgery. Preoperative CA19-9 was available for 10 patients with a median of 261 kU/L (26.5–1,733 kU/L). Preoperative imaging showed adjacent organ invasion into the liver (n = 18, 94.7%), hepatic vasculature (n = 7, 36.8%), hepatic flexure of the colon (n = 4, 21.1%), duodenum (n = 2, 10.5%), and pancreas (n = 1, 5.2%). Preoperative biliary drainage was performed in 9/15 (60.0%) of the patients with endoscopic retrograde cholangiography guided stent placement.
Two patients received neoadjuvant chemotherapy (NACT) due to locally advanced irresectable disease at presentation (Table 1). Out of nine patients who underwent a major hepatectomy alone, two also had a right hemicolectomy. A PD was performed without liver resection in two patients. Both these patients had a preoperative diagnosis of mid common bile duct cholangiocarcinoma, which was later confirmed as GBC on histopathology. One of these patients also required a right hepatic artery (RHA) resection and reconstruction. All patients were considered for adjuvant chemotherapy but only seven (36.8%) received it.
As shown in Table 2, the majority of tumors were associated with positive nodes (n = 14, 73.6%), moderate to poor grade (n = 15, 78.9%), peri neural (n = 15, 78.9%), and vascular invasion (n = 14, 73.6%).
Major postoperative complications occurred in 8/19 (42.1%) patients. The 30-day mortality was 4/19 (21.1%) (Table 3). These included one mortality after a major hepatectomy due to liver failure, two after a major hepatectomy with PD (HPD) due to sepsis and myocardial infarction each, and one after vascular resection and reconstruction due to sepsis.
Table 4 shows the characteristics of short and long term survivors. All eight short term survivors had preoperative jaundice with combined hepatic and bile duct involvement in 7/8 (87.5%) patients. Among these, six (75.0%) underwent a major HPD or vascular resection and reconstruction. One patient underwent curative resection despite isolated gastric metastasis (M1) which was detected intra operatively, due to their young age and good performance status. Among the long term survivors, 2/5 (40.0%) had preoperative jaundice and 4/5 (80.0%) underwent a major hepatectomy alone.
The estimated 2 year OS for the study cohort was 21%. The median OS in patients with and without jaundice at presentation was 4.1 months (0.78–11.1 months) and 13.7 months (12–30.4 months), respectively (p = 0.009). The estimated 2-year OS in these patients was 75% versus 7% (p = 0.008) (Fig. 1).
The median OS in patients who underwent either a major hepatectomy alone or minor HPD was significantly longer (11.3 months [6.8–17.3 months] versus 1.4 months [0.3–4.1 months]; p = 0.02) than patients who underwent HPD or vascular resection and reconstruction. The estimated 2 year OS in these patients was 33% and not reached (p = 0.010) (Fig. 2).
Locally advanced GBC is associated with ≤10% survival at one year [13]. The role of ER in these patients is not well defined. In the present study, despite high post-operative mortality (21.1%), five patients (26.3%) were alive > 12 months after resection. Negative surgical margins were achieved in 73.7% of patients, and ER in patients without preoperative jaundice was associated with an acceptable median survival of 13.7 months. Similarly, when ER was limited to major hepatectomy alone or minor HPD, median survival was 11.3 months.
Various patient and tumor related factors are associated with survival in patients with advanced GBC. Preoperative jaundice has been identified as a strong predictor of poor outcomes after resection [7,14,15]. Based on the results of a systematic review, jaundice in GBC is associated with irresectability, post-hepatectomy liver failure, bile leaks, and reduced survival [16]. However, surgical resection might improve survival in a subset of patients with jaundice when compared with no resection. Regimbeau et al. [17] reported a 1 and 3 year survival of 48% and 19%, respectively (postoperative mortality = 16%) in patients with jaundice who underwent resection. This was significantly better than a 1 and 3 year survival of 31% and 0%, respectively (p = 0.001) in patients without resection. In a recent Dutch multicenter analysis, Kuipers et al. [7] have shown that 8/9(88.9%) of short term survivors (< 6 months) after ER for GBC had preoperative jaundice. In the current study, all short term survivors (n = 8) had preoperative jaundice. The estimated 2-year OS was 75% versus 7% (p = 0.008) in patients without jaundice. However, 2/5 (40.0%) of the long term survivors (> 12 months) also had jaundice at the initial presentation. We believe that in addition to preoperative jaundice, the number of organs resected may have an impact on outcomes. Among the short term survivors, 7/8 (87.5%) had > 1 organ resection as opposed to 1/5 (20.0%) among the long term survivors (Table 3). It has been shown that in patients with advanced GBC, acceptable survival can be achieved if tumor extension is limited to one of the following: liver, hepato-duodenal ligament, and lymph nodes. When two or more of these organs are involved, mandating more aggressive resection, survival is poor [18]. We also noted that both short term and long term survival was better in patients who required either a major hepatectomy alone or a minor HPD. Major HPD and vascular resection and reconstruction was associated with short term survival and was required more frequently in patients with jaundice. Patients with GBC undergoing HPD have a high post-operative mortality (≤ 30%) and a 2-year survival as low as 0% [8,19]. Three patients underwent major HPD in the current study and did not survive beyond six months. Similarly, vascular involvement has been shown to be a strong predictor of survival in GBC [5]. Among four patients who underwent vascular resection and reconstruction, only one patient survived >12 months in the current study. This patient underwent surgery after demonstrating stable disease with NACT. Therefore major HPD and vascular resection and reconstruction might represent an acceptable option in advanced GBC, only if an objective response is seen with neoadjuvant treatment.
The role of chemotherapy, both in the neoadjuvant setting and as an adjuvant treatment modality, is being explored. While a favorable response and increased resectability has been demonstrated with NACT, its routine use remains debatable [20]. Similarly, adjuvant chemotherapy (BILCAP trial) or chemo radiation might improve survival, particularly in node-positive patients, but needs further validation [21,22].
There are certain limitations of the current study. Due to its retrospective design, some pertinent information might have been missed. The statistical significance of various associations like jaundice, combined hepatic and bile duct involvement, and the number of organs resected was not possible due to the small sample size. Also, the impact of NACT or adjuvant chemotherapy on outcomes could not be determined. Given that very few patients undergo ER for advanced GB cancer, it is difficult to determine the impact of chemotherapy in the neoadjuvant or adjuvant setting after ER, except in a multicenter setting.
In conclusion, despite high post-operative mortality, five (26.3%) patients survived >12 months after ER. Patients with poor outcomes had preoperative jaundice, combined hepatic and biliary involvement, and invasion of >1 adjacent organs. Therefore, acceptable survival can only be achieved with ERs in a select group of patients, after careful deliberation regarding high morbidity and mortality.
REFERENCES
1. Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, Wistuba II, et al. 2001; Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 51:349–364. DOI: 10.3322/canjclin.51.6.349. PMID: 11760569.

2. Stinton LM, Shaffer EA. 2012; Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 6:172–187. DOI: 10.5009/gnl.2012.6.2.172. PMID: 22570746. PMCID: PMC3343155.

3. Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. 2017; Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J Gastroenterol. 23:3978–3998. DOI: 10.3748/wjg.v23.i22.3978. PMID: 28652652. PMCID: PMC5473118.

4. Zhu AX, Hong TS, Hezel AF, Kooby DA. 2010; Current management of gallbladder carcinoma. Oncologist. 15:168–181. DOI: 10.1634/theoncologist.2009-0302. PMID: 20147507. PMCID: PMC3227945.

5. Yamamoto Y, Sugiura T, Ashida R, Okamura Y, Ito T, Uesaka K. 2017; Indications for major hepatectomy and combined procedures for advanced gallbladder cancer. Br J Surg. 104:257–266. DOI: 10.1002/bjs.10401. PMID: 27864927.

6. Chen C, Geng Z, Shen H, Song H, Zhao Y, Zhang G, et al. 2016; Long-term outcomes and prognostic factors in advanced gallbladder cancer: focus on the advanced T stage. PLoS One. 11:e0166361. DOI: 10.1371/journal.pone.0166361. PMID: 27846279. PMCID: PMC5112857.

7. Kuipers H, de Savornin Lohman EAJ, van Dooren M, Braat AE, Daams F, van Dam R, et al. 2021; Extended resections for advanced gallbladder cancer: results from a nationwide cohort study. Ann Surg Oncol. 28:835–843. DOI: 10.1245/s10434-020-08858-z. PMID: 32696306. PMCID: PMC7801314.

8. Sakamoto Y, Nara S, Kishi Y, Esaki M, Shimada K, Kokudo N, et al. 2013; Is extended hemihepatectomy plus pancreaticoduodenectomy justified for advanced bile duct cancer and gallbladder cancer? Surgery. 153:794–800. DOI: 10.1016/j.surg.2012.11.024. PMID: 23415082.

9. D'Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. 2009; Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 16:806–816. DOI: 10.1245/s10434-008-0189-3. PMID: 18985272.
10. Cai ZQ, Guo P, Si SB, Geng ZM, Chen C, Cong LL. 2017; Analysis of prognostic factors for survival after surgery for gallbladder cancer based on a Bayesian network. Sci Rep. 7:293. DOI: 10.1038/s41598-017-00491-3. PMID: 28331235. PMCID: PMC5428511.

11. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. 2017. AJCC cancer staging manual. 8th ed. Springer;Cham:
12. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. 2009; The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 250:187–196. DOI: 10.1097/SLA.0b013e3181b13ca2. PMID: 19638912.
13. Witjes CD, van den Akker SA, Visser O, Karim-Kos HE, de Vries E, Ijzermans JN, et al. 2012; Gallbladder cancer in the Netherlands: incidence, treatment and survival patterns since 1989. Dig Surg. 29:92–98. DOI: 10.1159/000336217. PMID: 22441693.

14. Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. 2004; Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 11:310–315. DOI: 10.1245/ASO.2004.03.011. PMID: 14993027.

15. Yang XW, Yuan JM, Chen JY, Yang J, Gao QG, Yan XZ, et al. 2014; The prognostic importance of jaundice in surgical resection with curative intent for gallbladder cancer. BMC Cancer. 14:652. DOI: 10.1186/1471-2407-14-652. PMID: 25187159. PMCID: PMC4164789.

16. Dasari BVM, Ionescu MI, Pawlik TM, Hodson J, Sutcliffe RP, Roberts KJ, et al. 2018; Outcomes of surgical resection of gallbladder cancer in patients presenting with jaundice: a systematic review and meta-analysis. J Surg Oncol. 118:477–485. DOI: 10.1002/jso.25186. PMID: 30259519.

17. Regimbeau JM, Fuks D, Bachellier P, Le Treut YP, Pruvot FR, Navarro F, et al. 2011; Prognostic value of jaundice in patients with gallbladder cancer by the AFC-GBC-2009 study group. Eur J Surg Oncol. 37:505–512. DOI: 10.1016/j.ejso.2011.03.135. PMID: 21514090.

18. Miura F, Asano T, Amano H, Toyota N, Wada K, Kato K, et al. 2010; New prognostic factor influencing long-term survival of patients with advanced gallbladder carcinoma. Surgery. 148:271–277. DOI: 10.1016/j.surg.2010.04.022. PMID: 20570306.

19. Kaneoka Y, Yamaguchi A, Isogai M. 2007; Hepatopancreatoduodenectomy: its suitability for bile duct cancer versus gallbladder cancer. J Hepatobiliary Pancreat Surg. 14:142–148. DOI: 10.1007/s00534-006-1108-2. PMID: 17384904.

20. Hakeem AR, Papoulas M, Menon KV. 2019; The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - a systematic review. Eur J Surg Oncol. 45:83–91. DOI: 10.1016/j.ejso.2018.08.020. PMID: 30287098.

21. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. 2019; Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 20:663–673. Erratum in: Lancet Oncol 2019;20:e242.

22. Kim TH, Woo SM, Lee WJ, Oh ES, Youn SH, Moon SH, et al. 2019; Benefit of adjuvant chemoradiotherapy in resected gallbladder carcinoma. Sci Rep. 9:11770. DOI: 10.1038/s41598-019-48099-z. PMID: 31409811. PMCID: PMC6692378.

Fig. 1
Estimated 2-year overall survival after extended resection for gallbladder cancer based on the presence (n = 4) or absence (n = 15) of preoperative jaundice.
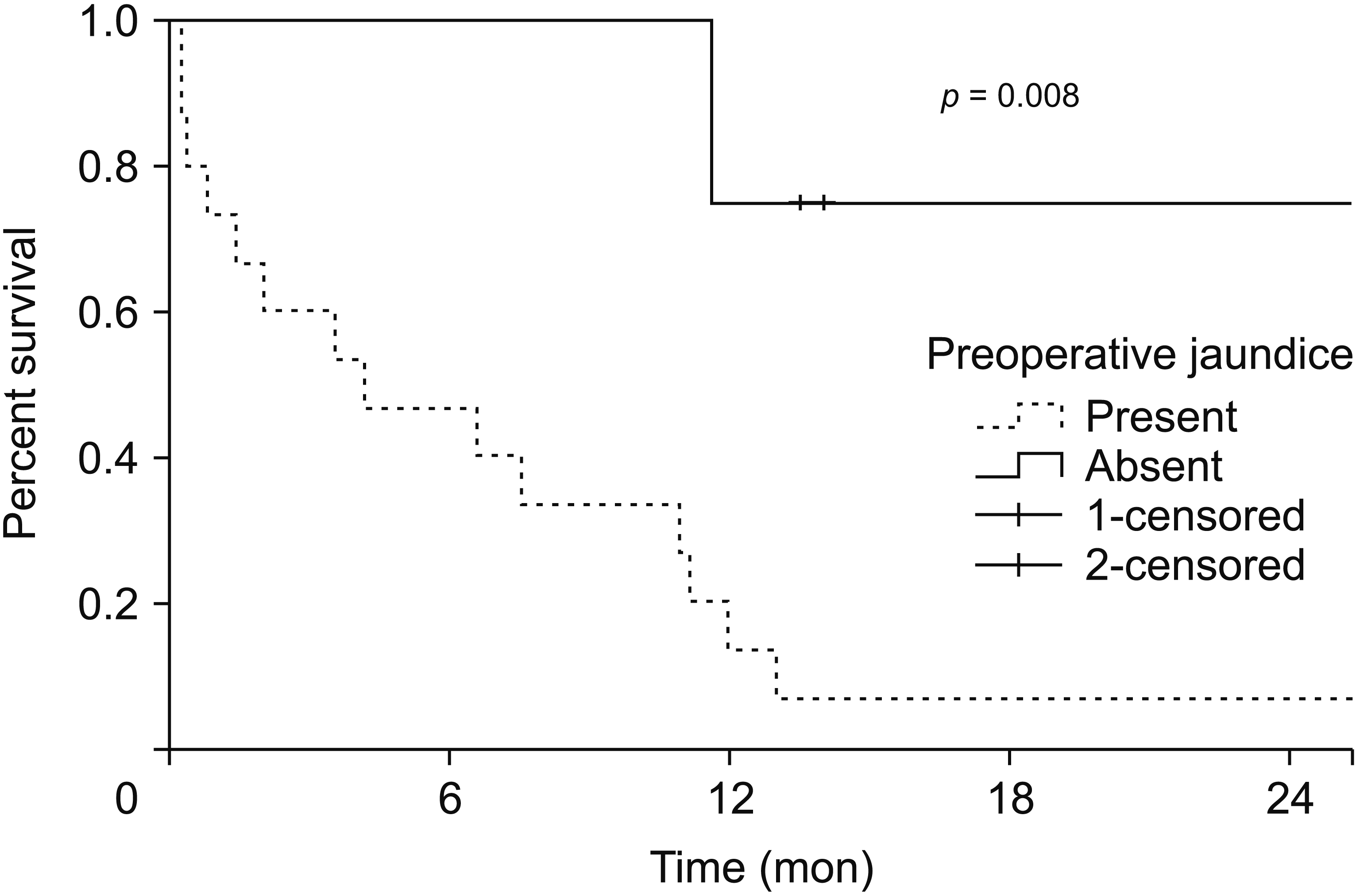
Fig. 2
Estimated 2-year overall survival with types of extended resection for gallbladder cancer (major HPD or vascular resection [n = 9] versus major hepatectomy alone or minor HPD [n = 10]). PD, pancreaticoduodenectomy; HPD, hepatectomy with PD.
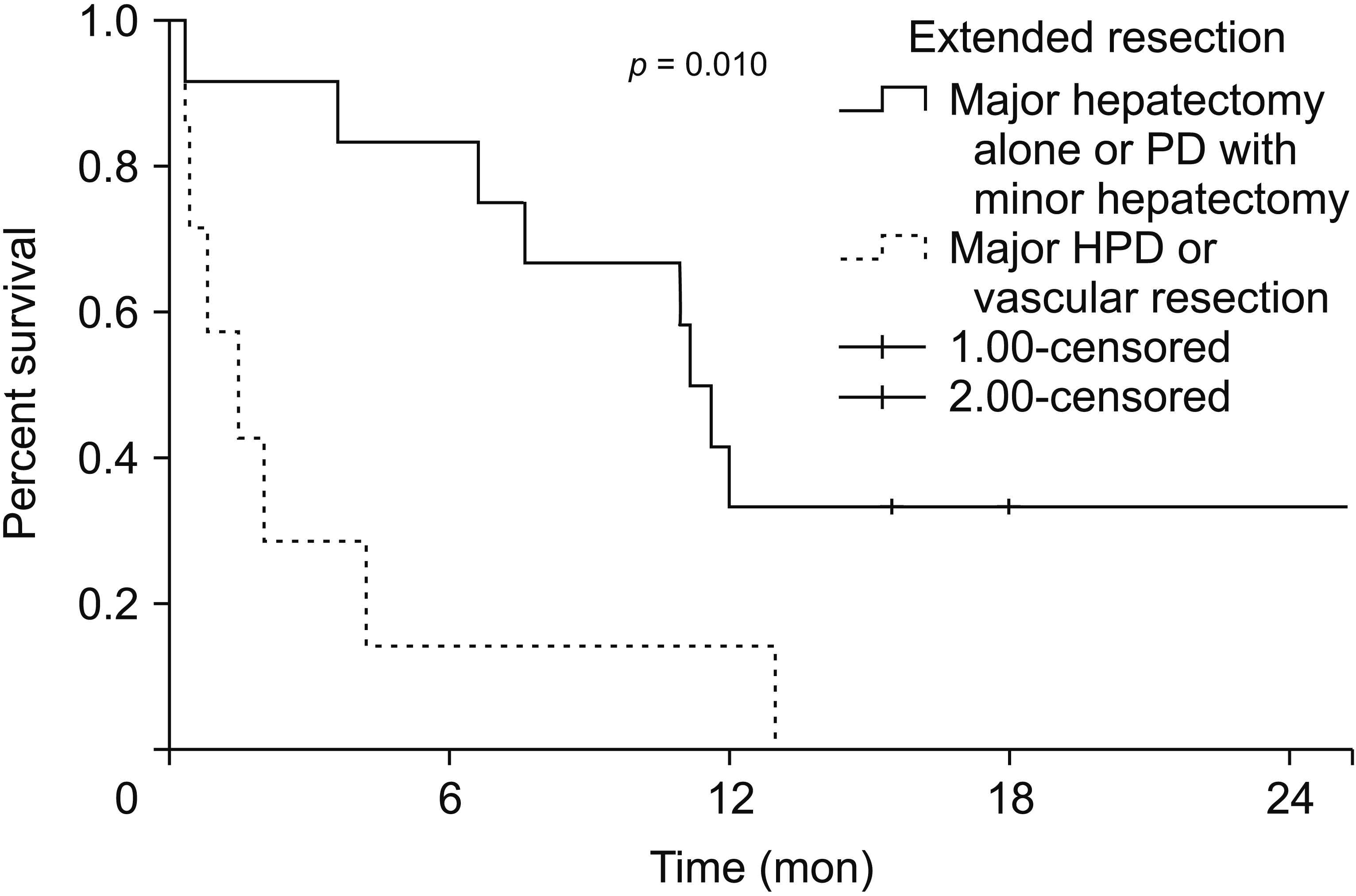
Table 1
Patient characteristics and treatment received
Table 2
Histopathological characteristics of patients who underwent extended resections for gallbladder cancer
Table 3
Postoperative morbidity and in hospital mortality in patients with gallbladder cancer after extended resections
Table 4
Characteristics of short term (< 6 months) and long term survivors (> 12 months) after extended resection for gallbladder cancer




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download