Abstract
Backgrounds/Aims
To evaluate surgical outcomes of patients with gallbladder wall thickness (GBWT) > 5 mm.
Methods
Patients who underwent cholecystectomy were classified into two groups according to their GBWT status (GBWT+ vs. GBWT–).
Results
Among 1,211 patients who underwent cholecystectomy, GBWT+ was seen in 177 (14.6%). The GBWT+ group was significantly older with more males, higher ASA score, higher alkaline phosphatase level, higher international normalized ratio, and lower albumin level than the GBWT– group. On ultrasound, GBWT+ patients had larger stone size, more pericholecystic fluid, more common bile duct stone, and more biliary pancreatitis. Compared with the GBWT– group, the GBWT+ group had more urgent surgeries (12.4% vs. 3.2%, p = 0.001), higher conversion rate (4.5% vs. 0.3%, p = 0.001), prolonged operative time (67 ± 38 vs. 54 ± 29 min; p = 0.001), more bleeding (3.4% vs. 0.5%, p = 0.002), and more need of drain (21.5% vs. 10.5%, p = 0.001). By multivariate analysis, factors associated with increased length of hospital stay were GBWT+ (HR: 1.97, 95% CI: 1.19–3.25, p = 0.008), urgent surgery (HR: 10.2, 95% CI: 4.07–25.92, p = 0.001), prolonged surgery (HR: 1.01, 95% CI: 1.0–1.02, p = 0.001), and postoperative drain (HR: 11.3, 95% CI: 6.40–20.0, p = 0.001).
There are many reasons to predict a potentially complicated cholecystectomy in a dynamic clinical setting. It is crucial to know if a patient is a candidate for a one-day outpatient procedure or if they need to be treated with an inpatient procedure. Additionally, the appropriate time and procedure length must be determined to avoid overloading the hospital’s infrastructure. The patient and the surgical team must be prepared for a possible conversion and the trainees or senior surgeons must consent to perform the procedure. The increased gallbladder wall thickness (GBWT) can negatively impact the outcome of a cholecystectomy. GBWT is an independent factor associated with complex surgeries and is included in many predicting preoperative scores. Most researchers use a model where a GBWT > 3 mm or > 4 mm is considered a predictor of increased complications [1-7]. We relatively notice, as with other study [8], a straightforward cholecystectomy when the wall thickness is less than 5 mm. We hypothesized that a GBWT equal to or more than 5 mm was associated with a higher likelihood of postoperative complications. Our study aims to evaluate the surgical outcome in patients with a GBWT > 5 mm, regarding operative time, conversion to open, intraoperative, and postoperative complications. The secondary goal was to assess any relation of GBWT to hospitalization time.
Patient evaluation and data collection of GBWT were prospectively performed at a single, private tertiary center, Dr. Soliman Fakeeh Hospital (Jeddah, Saudi Arabia). The current study investigated the impact of GBWT in 1,211 consecutive patients who underwent gallbladder operation in our institution between August 2018 and August 2021.
We excluded pediatric patients and patients who had a cholecystectomy combined with other surgeries. According to radiological findings on GBWT, the patients were classified into two groups, those with wall thickness equal to or more than 5 mm, defined as GBWT+ and those with wall thickness less than 5 mm, GBWT–.
The GBWT was confirmed by ultrasonography (US), computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), and diagnostic laparoscopy. Since there was no significant difference between the US, pathology measurement of GBWT, and evidence of tissue shrinkage post-resection in formalin, we used the preoperative clinical evaluation rather than the histopathology [9-11]. The measured outcome included surgical intervention, conversion to open, complication rate, and additional assessment of the factors that prolonged inpatient hospital stay postoperatively.
The Institutional Review Board of Dr. Soliman Fakeeh Hospital approved the study protocol; approval No. 228/IRB/2021. The informed consent was waived by instituitonal ethical committee.
Standard laparoscopic cholecystectomy is the procedure of choice in our institution to manage gallbladder disease. However, the surgeons' preference and clinical state determine whether to perform an open approach or convert from laparoscopy. Since the study was conducted in a private hospital, all surgeries were performed by qualified expert consultant-level surgeons.
The demographic and clinical variables of the two groups were compared using Fisher’s exact test with two-sided verification and Pearson’s χ2 test or an unpaired Student’s t-test, depending on the nature of the data. In addition, a linear regression model was created with a backward stepwise approach to select the factors that independently affected the length of the stay, followed by multivariate logistic regression and measuring the odds ratio (OR) with a 95% confidence interval (CI). A p-value of less than 0.05 was considered statistically significant. Data were analyzed using SPSS software (version 25; IBM Corp., Armonk, NY, USA).
Of the 1,211 patients who underwent cholecystectomy, 177 were GBWT+ (14.6%). The GBWT+ group was older than the GBWT– with a mean age of 45 ± 14.6 years vs. 41 ± 13.5 years, respectively (p = 0.001). Compared to the GBWT–, GBWT+ patients were mostly male (39.0% vs. 29.3%, p = 0.013), with a high American society of anesthesiologists score (1.7% vs. 0.1%, p = 0.011), high alkaline phosphatase U/I 120 ± 102 vs. 94 ± 57 (p = 0.005), high international normalized ratio (INR) (1.3 ± 2.7 vs. 0.9 ± 0.2; p = 0.007), and a low preoperative albumin level (3.2 ± 0.5 g/dL vs. 3.6 ± 0.6 g/dL; p = 0.017). On radiological investigation, GBWT+ had significant association, in contrast to GBWT–, with stone size > 5 mm (57.1% vs. 42.1%; p = 0.001), pericholecystic fluid (13.0% vs. 2.2%; p = 0.001), and common bile duct (CBD) stones (9.0% vs. 3.7%, p = 0.004). It was significantly more probable for the GBWT+ group to require preoperative endoscopic retrograde cholangiopancreatography (ERCP) and experience biliary pancreatitis. GBWT+ group was statistically more likely to need preoperative ERCP and suffer from biliary pancreatitis.
There was no statistically significant difference between both groups concerning body mass index body mass index (BMI), diabetes mellitus, comorbidities, smoking, weight loss, duration of symptoms, leukocyte, liver function test, total bilirubin, and serum creatinine (Table 1).
Urgent surgery was more common with GBWT+ than with GBWT– patients (12.4% vs. 3.2%; p = 0.001). GBWT+ patients had a higher conversion rate (4.5% vs. 0.3%; p = 0.001), longer operative times (67 ± 38 vs. 54 ± 29 min; p = 0.001), intraoperative bleeding >100 mL (3.4% vs. 0.5%; p = 0.002), gangrenous gallbladder (5.6% vs. 0.8%; p = 0.001), and need for drain (21.5% vs. 10.5%; p = 0.001). The postoperative collection, stump leak, wound infection, and the need for reoperation were not statistically different between the two groups. One GBWT– patient had a CBD injury (1/1,211, 0.082%), which required hepaticojejunostomy reconstruction (Table 1).
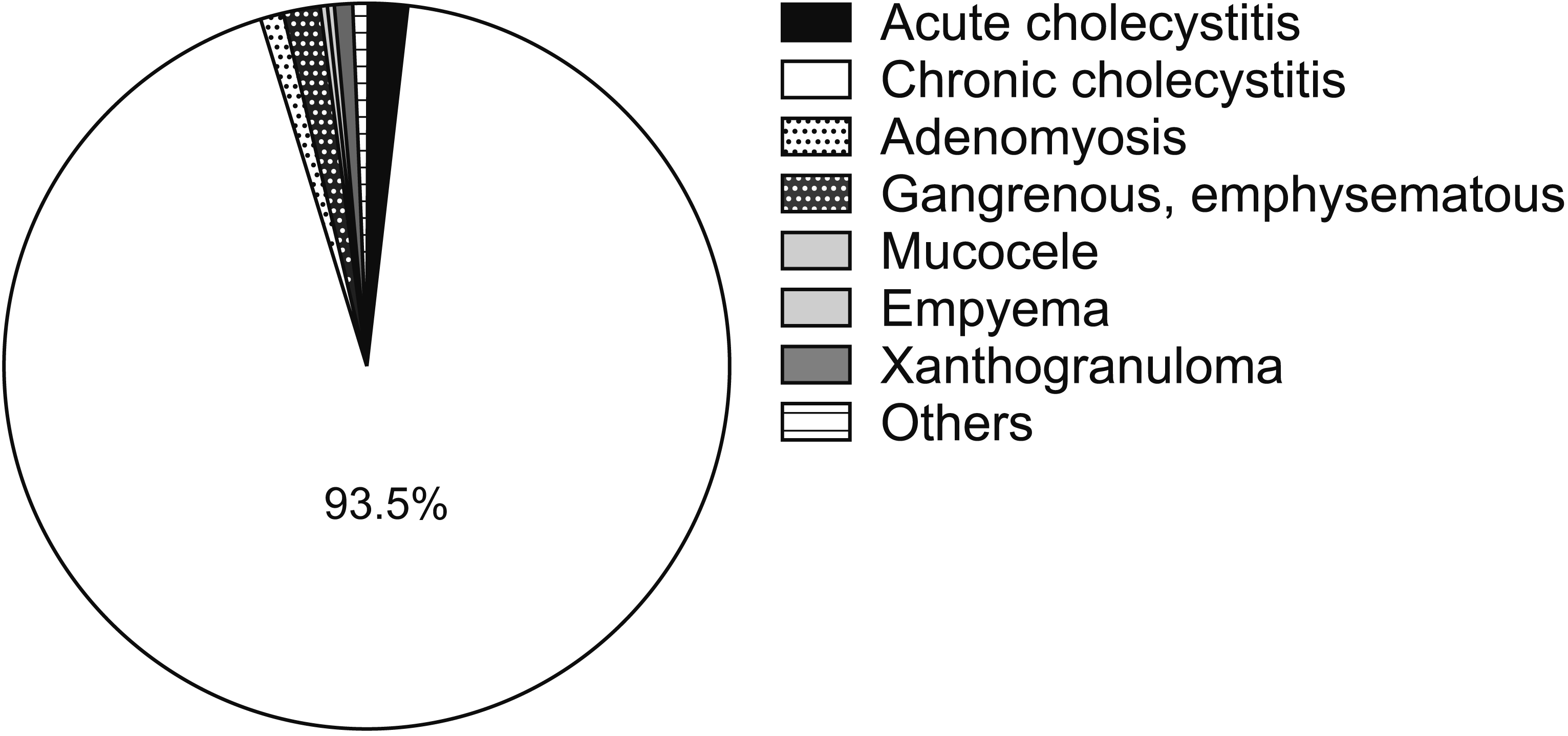
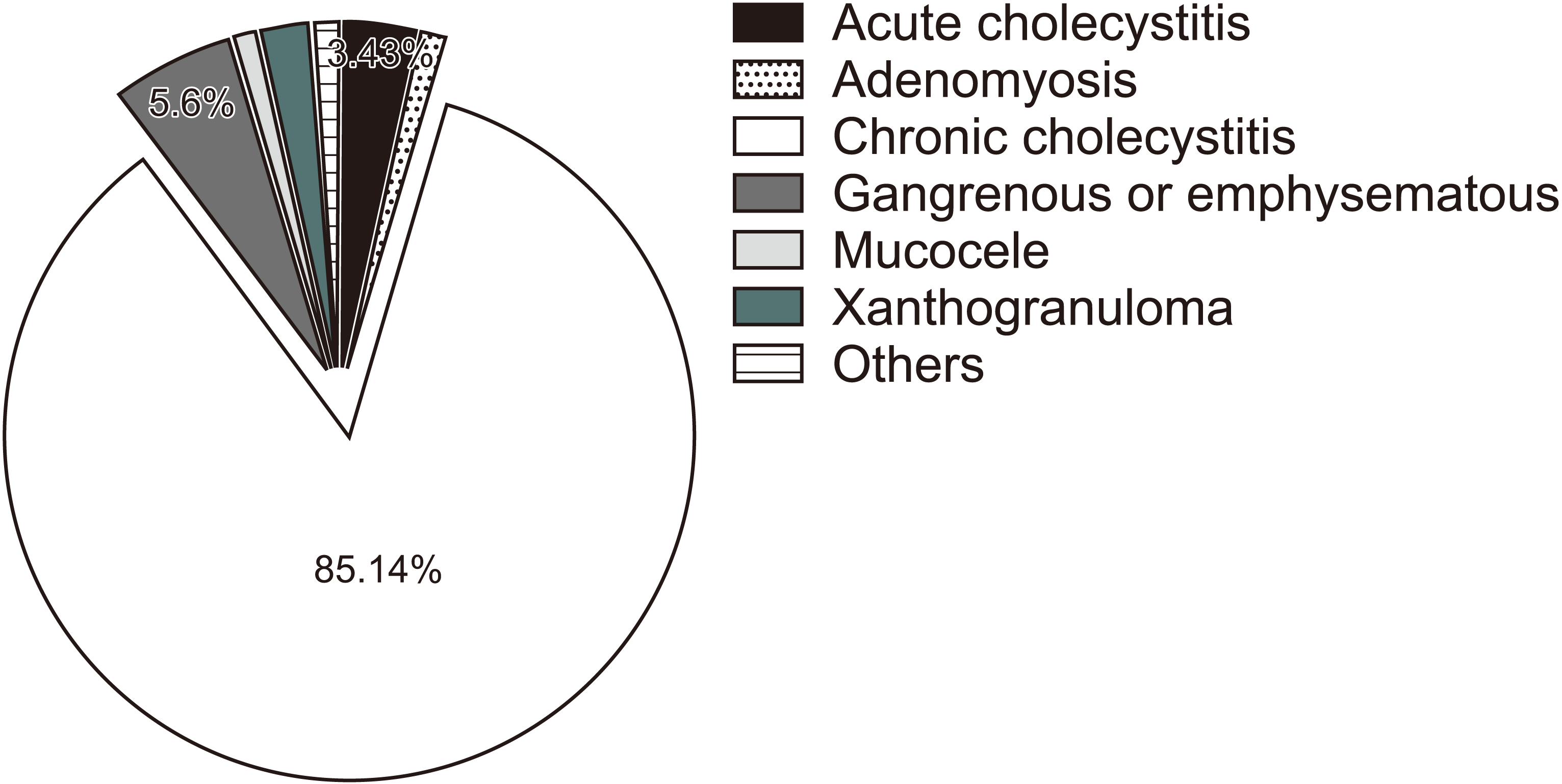
Most of the specimens were chronic or acute on top of chronic cholecystitis (93.5%); the remaining were acute cholecystitis, adenomyosis, gangrenous, mucocele, xanthogranuloma, and lymphoma. None of the histopathologies revealed an incidental finding of a malignant tumor or dysplasia (Fig. 1). The GBWT+ group had more findings of acute cholecystitis (3.4% vs. 1.5%), gangrenous gallbladder (5.7% vs. 0.8%, mucocele 1.1% vs 0.1%, xanthogranuloma 2.3% vs. 0.6%). On the other hand, the GBWT– group had more findings of chronic cholecystitis (94.9% vs. 85.9%). After histopathology was categorized into two tiers, chronic cholecystitis versus others, the GBWT+ patients had significantly more of other histopathology findings (14.1% vs. 5.1%; p = 0.001) (Fig. 2).
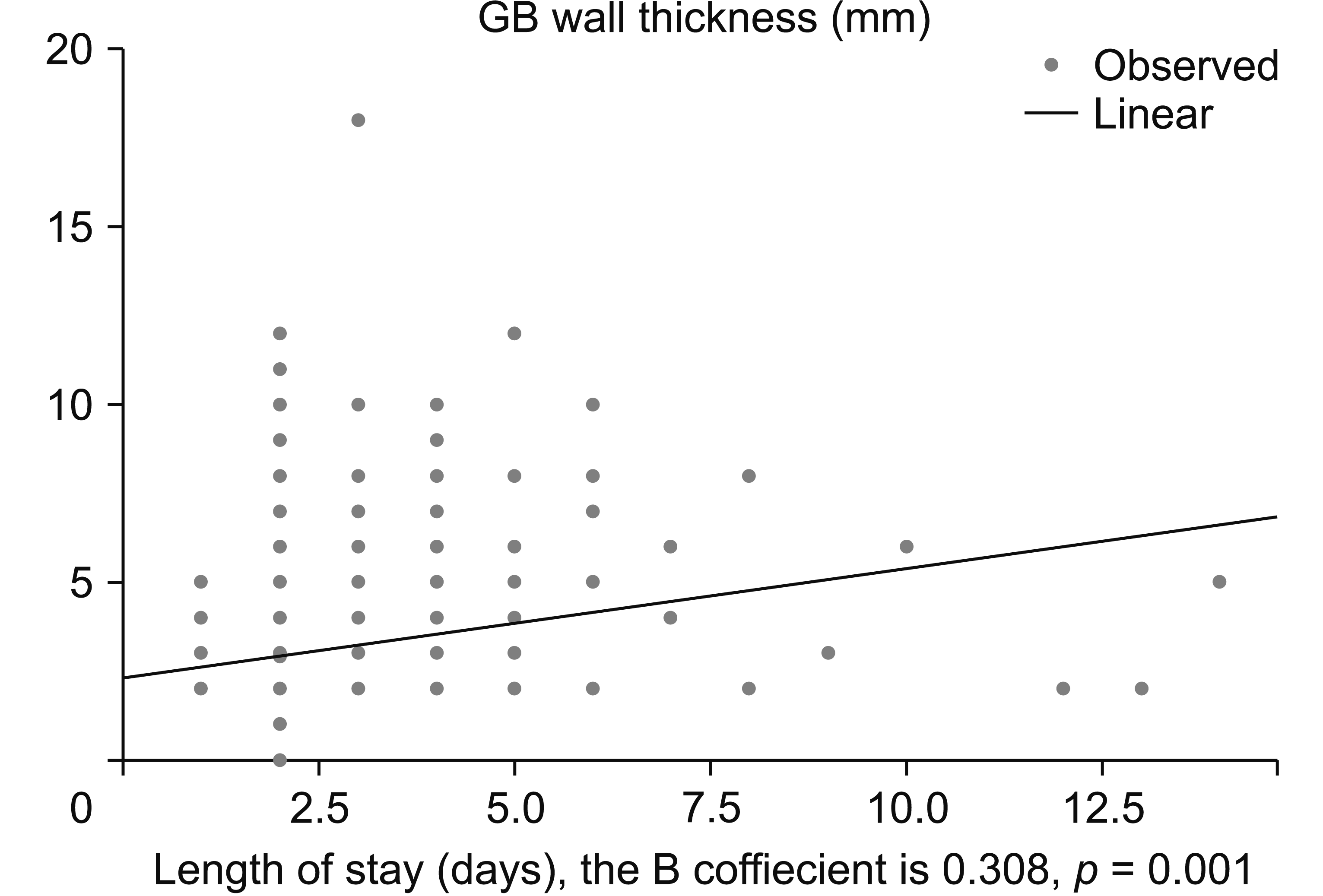
After building the linear regression model, the statistically significant predictors of increased length of stay of more than 72 hours were age, duration of symptoms, INR, GBWT+, urgent surgery, prolonged operative time, postoperative drain, and collection. The significant independent predictors of prolonged hospitalization on binary logistic regression were GBWT+ (hazard ratio [HR] = 1.97, 95% CI = 1.19–3.25, p = 0.008), urgent surgery (HR = 10.2, 95% CI = 4.07–25.92; p = 0.001), operative time > 60 min (HR = 1.01, 95% CI = 1.0–1.02, p = 0.001), and postoperative drain (HR = 11.3, 95% CI = 6.40–20.0, p = 0.001) (Table 2, Fig. 3).
GBWT increases in many diseases, such as nonalcoholic fatty liver, sclerosing cholangitis, autoimmune pancreatitis, and incidental gallbladder cancer (GBC) [12,13]. GBWT increases is an independent predictor of GBC (OR = 4.39, p = 0.0003), and should be carefully interpreted [14-17]. Studies suggested that using GBWT as a noninvasive predictor of gastroesophageal varices in cirrhotic and portal hypertension patients [18]. Other reports investigate GBWT as a marker of bleeding risk in patients with dengue fever [19]. The surgeon has a 14.6% chance, based on our data, of operating a patient with a GBWT > 5 mm. In a recent metanalysis that included 4,194 patients, GBWT predicted failure of outpatient surgery (OR, 2.89; 95% CI, 1.34–4.04; p = 0.003) [20]. In the literature, a group of variables was consistently seen as predictors for increased conversion rate, intraoperative events, postoperative complications, and extended hospital stay. These variables included age, male patients, BMI, American society of anesthesiologist score, GBWT, operative time, CBD stones, preoperative ERCP, and urgent surgery [1-5,7,20]. In line with Kokoroskos et al. [21], the length of symptoms in our group did not predict GBWT, surgical difficulties, or length of stay; as a result, GBWT can take the place of the conventional complaint time to predict surgical outcome. Our analysis showed a significant association of GBWT > 5 mm with increased age, male gender, high ASA, INR, and alkaline phosphatase.
We noticed, as in other studies [22], that low preoperative albumin levels in the GBWT+ group could play a role in postoperative morbidity in surgical patients. On the contrary, some studies found no correlation between wall thickness and albumin level [23,24]. The GBWT+ group had a higher tendency to bleed intraoperatively. A potential explanation could be this group's high INR level or the companion's thickened wall hyperemia, but further evidence is needed. However, there was no difference in the liver enzymes between both groups; hypoalbuminemia, high INR, and alkaline phosphatase could result from hepatic dysfunction in GBWT+ patients.
The conversion rate in this review was 0.9% (11/1,211) with GBWT+ vs. GBWT– rate of 4.5% vs. 0.3%, p = 0.001, which is low compared to 8.2% and 7.8% in other reports [21,25]. The reason for the relatively low conversion rate and major events, such as CBD injury, was justified by the surgeon’s high level of experience. Our institution is a private sector and senior surgeons perform the procedures. In cases of acute cholecystitis, postoperative drain insertion was not part of our standard procedure. One-fifth of the GBWT+ group required a drain, which negatively reflected the length of hospitalization in concordance with what was concluded in a systemic review of 1,274 patients [26]. Despite the adverse effect of GBWT+ on the discharge plan, other factors also play a role. The urgency of surgery and postoperative drain were the leading cause of delayed discharge of more than 72 hours in our cohort.
It was unclear why some patients were GBWT+ compared to others. In our final histopathology, chronic cholecystitis represented 93% of the sample; only 14.7% of patients were GBWT+. The theory of genetic variation between patients can explain such phenomena. There is a report linking GBWT+ in Mexican Americans to markers D11S912 and D11S968 on chromosome 11q24-q25 [27]. More evidence is required before using such a finding in clinical practice.
It was unusual that we did not record any incidental cancer cases. The incidental GBC in the literature was 0.36% (0.19%–1.65%) based on a systemic review of ten studies [14]. Holanda et al. [28] reported six patients of GBC (0.5%) in a sample of 1,251 cholecystectomies, which was similar to our sample size. The relatively young population in our study can be the reason. One specimen was detected to have a lymphoid follicle with a positive BCL2, CD3, and CD20 immunohistochemistry stain, and diagnosis of BCL lymphoma was confirmed incidentally from gallbladder tissue.
In our cohort of 1,211 cholecystectomies, 98.8% (1,197) were laparoscopically performed. Our study had no variability among surgeons since all were qualified at the consultant level. Our institution plans to start a training program for residents in the coming few years. Another point was the young average age of the samples, which reflects our country's population's youth. A potential limitation could be the lack of randomization and the subjective assessment of GBWT by radiologists.
In conclusion, GBWT unfavorably affects the surgical outcome. Variables such as GBWT ≥ 5 mm, urgent prolonged operation, and postoperative drains are independent predictors of an extended hospital stay. Patients with GBWT+ were more likely to experience complications and have a higher likelihood of spending more than 72 hours in the hospital. Therefore, allocating an expert team to operate on patients with GBWT+ was warranted. GBWT+ might indicate the patient or the healthcare provider to consider extra hospitalization time.
ACKNOWLEDGEMENTS
The author would like to express his deep gratitude to the IRB committee in Dr. Soliman Fakeeh Hospital, Jeddah, for accepting the design and providing the data for this review.
REFERENCES
1. Nassar AHM, Hodson J, Ng HJ, Vohra RS, Katbeh T, Zino S, et al. 2020; Predicting the difficult laparoscopic cholecystectomy: development and validation of a pre-operative risk score using an objective operative difficulty grading system. Surg Endosc. 34:4549–4561. DOI: 10.1007/s00464-019-07244-5. PMID: 31732855.
2. Gupta N, Ranjan G, Arora MP, Goswami B, Chaudhary P, Kapur A, et al. 2013; Validation of a scoring system to predict difficult laparoscopic cholecystectomy. Int J Surg. 11:1002–1006. DOI: 10.1016/j.ijsu.2013.05.037. PMID: 23751733.

3. Randhawa JS, Pujahari AK. 2009; Preoperative prediction of difficult lap chole: a scoring method. Indian J Surg. 71:198–201. DOI: 10.1007/s12262-009-0055-y. PMID: 23133154. PMCID: PMC3452633.

4. Siddiqui MA, Rizvi SAA, Sartaj S, Ahmad I, Rizvi SWA. 2017; A standardized ultrasound scoring system for preoperative prediction of difficult laparoscopic cholecystectomy. J Med Ultrasound. 25:227–231. DOI: 10.1016/j.jmu.2017.09.001. PMID: 30065497. PMCID: PMC6029324.

5. Rosen M, Brody F, Ponsky J. 2002; Predictive factors for conversion of laparoscopic cholecystectomy. Am J Surg. 184:254–258. DOI: 10.1016/S0002-9610(02)00934-0. PMID: 12354595.

6. Nachnani J, Supe A. 2005; Pre-operative prediction of difficult laparoscopic cholecystectomy using clinical and ultrasonographic parameters. Indian J Gastroenterol. 24:16–18.
7. Ishizaki Y, Miwa K, Yoshimoto J, Sugo H, Kawasaki S. 2006; Conversion of elective laparoscopic to open cholecystectomy between 1993 and 2004. Br J Surg. 93:987–991. DOI: 10.1002/bjs.5406. PMID: 16739098.

8. Kania D. 2016; Ultrasound measurement of the gallbladder wall thickness in the assessment of the risk of conversion from elective laparoscopic cholecystectomy to open surgery - Olkusz county experience. Pol Przegl Chir. 88:334–345. DOI: 10.1515/pjs-2016-0073. PMID: 28141556.

9. Cefalu L, McMurray R, Sizemore G, Bieniek G, Lustik M, Yheulon C. 2019; Accuracy of right upper quadrant ultrasound in estimating gallbladder wall thickness. Surg Laparosc Endosc Percutan Tech. 29:26–30. DOI: 10.1097/SLE.0000000000000580. PMID: 30520813.

10. Engel JM, Deitch EA, Sikkema W. 1980; Gallbladder wall thickness: sonographic accuracy and relation to disease. AJR Am J Roentgenol. 134:907–909. DOI: 10.2214/ajr.134.5.907. PMID: 6768264.

11. Pereira-Sampaio MA, Marques-Sampaio BP, Sampaio FJ, Henry RW. 2011; Shrinkage of renal tissue after impregnation via the cold Biodur plastination technique. Anat Rec (Hoboken). 294:1418–1422. DOI: 10.1002/ar.21432. PMID: 21714112.

12. Watanabe K, Kamisawa T, Chiba K, Kikuyama M, Nakahodo J, Igarashi Y. 2021; Gallbladder wall thickening in patients with IgG4-related diseases, with special emphasis on IgG4-related cholecystitis. Scand J Gastroenterol. 56:1456–1461. DOI: 10.1080/00365521.2021.1971758. PMID: 34486468.

13. Colak Y, Bozbey G, Erim T, Caklili OT, Ulasoglu C, Senates E, et al. 2016; Impaired gallbladder motility and increased gallbladder wall thickness in patients with nonalcoholic fatty liver disease. J Neurogastroenterol Motil. 22:470–476. DOI: 10.5056/jnm15159. PMID: 26932908. PMCID: PMC4930302.

14. Kellil T, Chaouch MA, Aloui E, Tormane MA, Taieb SK, Noomen F, et al. 2021; Incidence and preoperative predictor factors of gallbladder cancer before laparoscopic cholecystectomy: a systematic review. J Gastrointest Cancer. 52:68–72. DOI: 10.1007/s12029-020-00524-7. PMID: 32964323.

15. Goussous N, Maqsood H, Patel K, Ferdosi H, Muhammad N, Sill AM, et al. 2018; Clues to predict incidental gallbladder cancer. Hepatobiliary Pancreat Dis Int. 17:149–154. DOI: 10.1016/j.hbpd.2018.02.001. PMID: 29709218.

16. Zhu JQ, Han DD, Li XL, Kou JT, Fan H, He Q. 2015; Predictors of incidental gallbladder cancer in elderly patients. Hepatobiliary Pancreat Dis Int. 14:96–100. DOI: 10.1016/S1499-3872(14)60292-7. PMID: 25655297.

17. Koshenkov VP, Koru-Sengul T, Franceschi D, Dipasco PJ, Rodgers SE. 2013; Predictors of incidental gallbladder cancer in patients undergoing cholecystectomy for benign gallbladder disease. J Surg Oncol. 107:118–123. DOI: 10.1002/jso.23239. PMID: 22886779.

18. Elkerdawy MA, Ahmed MH, Zaghloul MS, Haseeb MT, Emara MH. 2021; Does gallbladder wall thickness measurement predict esophageal varices in cirrhotic patients with portal hypertension? Eur J Gastroenterol Hepatol. 33:917–925. DOI: 10.1097/MEG.0000000000002024. PMID: 33908388.

19. Asghar MS, Yasmin F, Tahir MJ, Anwar S, Yaseen R, Yousaf Z. 2022; Predictive analysis of gallbladder wall thickness as a marker for bleeding risk and need for transfusion in dengue patients. Jpn J Infect Dis. 75:234–240. DOI: 10.7883/yoken.JJID.2021.076. PMID: 34588363.

20. Balciscueta I, Barberà F, Lorenzo J, Martínez S, Sebastián M, Balciscueta Z. 2021; Ambulatory laparoscopic cholecystectomy: systematic review and meta-analysis of predictors of failure. Surgery. 170:373–382. DOI: 10.1016/j.surg.2020.12.029. PMID: 33558068.

21. Kokoroskos N, Peponis T, Lee JM, El Hechi M, Naar L, Elahad JA, et al. 2020; Gallbladder wall thickness as a predictor of intraoperative events during laparoscopic cholecystectomy: a prospective study of 1089 patients. Am J Surg. 220:1031–1037. DOI: 10.1016/j.amjsurg.2020.03.007. PMID: 32178838.

22. Ralls PW, Quinn MF, Juttner HU, Halls JM, Boswell WD. 1981; Gallbladder wall thickening: patients without intrinsic gallbladder disease. AJR Am J Roentgenol. 137:65–68. DOI: 10.2214/ajr.137.1.65. PMID: 6787892.

23. Kaftori JK, Pery M, Green J, Gaitini D. 1987; Thickness of the gallbladder wall in patients with hypoalbuminemia: a sonographic study of patients on peritoneal dialysis. AJR Am J Roentgenol. 148:1117–1118. DOI: 10.2214/ajr.148.6.1117. PMID: 3554931.

24. Bremer SCB, Knoop RF, Porsche M, Amanzada A, Ellenrieder V, Neesse A, et al. 2022; Pathological gallbladder wall thickening is associated with advanced chronic liver disease and independent of serum albumin. J Clin Ultrasound. 50:367–374. DOI: 10.1002/jcu.23077. PMID: 34633098.

25. Raman SR, Moradi D, Samaan BM, Chaudhry US, Nagpal K, Cosgrove JM, et al. 2012; The degree of gallbladder wall thickness and its impact on outcomes after laparoscopic cholecystectomy. Surg Endosc. 26:3174–3179. DOI: 10.1007/s00464-012-2310-8. PMID: 22538700.

26. Cirocchi R, Kwan SH, Popivanov G, Ruscelli P, Lancia M, Gioia S, et al. 2021; Routine drain or no drain after laparoscopic cholecystectomy for acute cholecystitis. Surgeon. 19:167–174. DOI: 10.1016/j.surge.2020.04.011. PMID: 32713729.

27. Samudrala N, Farook VS, Dodd GD, Puppala S, Schneider J, Fowler S, et al. 2008; Autosomal genome-wide linkage analysis to identify loci for gallbladder wall thickness in Mexican Americans. Hum Biol. 80:11–28. DOI: 10.3378/1534-6617(2008)80[11:AGLATI]2.0.CO;2. PMID: 18505042.

28. Holanda AKG, Lima Júnior ZB. 2020; Gallbladder histological alterations in patients undergoing cholecystectomy for cholelithiasis. Rev Col Bras Cir. 46:e20192279. DOI: 10.1590/0100-6991e-20192279. PMID: 31967243.
Table 1
Demographic and clinical features of the study patients according to GBWT status
| Variable | GBWT (+) (n = 177) | GBWT (–) (n = 1,034) | p-valuea) |
|---|---|---|---|
| Age at surgery (yr) | 45 ± 14.6 | 41 ± 13.5 | 0.001 |
| Body mass index (kg/m2) | 30.7 ± 6.5 | 30.1 ± 6.5 | 0.29 |
| Sex | 0.013 | ||
| Male | 69 (39.0) | 303 (29.3) | |
| Female | 108 (61.0) | 731 (70.7) | |
| ASA score | 0.011 | ||
| I + II +III | 174 (98.3) | 1,032 (99.9) | |
| IV + V | 3 (1.7) | 1 (0.1) | |
| Diabetes | 0.24 | ||
| Yes | 37 (20.9) | 178 (17.2) | |
| No | 140 (79.1) | 856 (82.8) | |
| Comorbidity | 0.35 | ||
| Yes | 84 (47.5) | 530 (51.3) | |
| No | 93 (52.5) | 504 (48.7) | |
| Smoking | 0.57 | ||
| Yes | 37 (20.9) | 236 (22.8) | |
| No | 140 (79.1) | 798 (77.2) | |
| Weight loss | 0.49 | ||
| Yes | 20 (11.3) | 136 (13.2) | |
| No | 157 (88.7) | 898 (86.8) | |
| Duration of complaint (wk) | 13.5 ± 47 | 19.7 ± 46 | 0.14 |
| WBC (µ/L) | 8.6 ± 4.2 | 8.5 ± 16 | 0.94 |
| HB (g/dL) | 12.9. ± 2.7 | 13.3 ± 5.6 | 0.45 |
| Albumin (g/dL) | 3.2 ± 0.5 | 3.6 ± 0.6 | 0.017 |
| Total bilirubin (mg/dL) | 0.9 ± 1.1 | 0.8 ± 1.8 | 0.56 |
| Alkaline phosphatase (U/I) | 120 ± 102 | 94 ± 57 | 0.005 |
| SGOT/AST (U/I) | 60 ± 117 | 59 ± 115 | 0.93 |
| SGPT/ALT (U/I) | 89 ± 144 | 72 ± 147 | 0.21 |
| Creatinine (mg/dL) | 2.4 ± 12.2 | 1.8 ± 12 | 0.60 |
| INR | 1.3 ± 2.7 | 0.9 ± 0.2 | 0.007 |
| Stones size > 5 mm | 0.001 | ||
| Yes | 101 (57.1) | 436 (42.1) | |
| No | 53 (29.9) | 434 (41.9) | |
| Stone size (mm) | 10 ± 8 | 9 ± 7 | 0.028 |
| Pericholecystic fluid | 0.001 | ||
| Yes | 23 (13.0) | 23 (2.2) | |
| No | 154 (87.0) | 931 (90.0) | |
| GB wall thickness (mm) | 6.3 ± 2 | 2.4 ± 0.7 | 0.001 |
| CBD diameter (mm) | 4.9 ± 2.3 | 4.1 ± 1.5 | 0.001 |
| CBD stone | 0.004 | ||
| Yes | 16 (9.0) | 38 (3.7) | |
| No | 161 (91.0) | 996 (96.3) | |
| Biliary pancreatitis | 0.001 | ||
| Yes | 17 (9.6) | 35 (3.4) | |
| No | 160 (90.4) | 999 (96.6) | |
| Preoperative ERCP | 0.001 | ||
| Yes | 33 (18.6) | 76 (7.4) | |
| No | 144 (81.4) | 955 (92.6) | |
| Surgery sitting | 0.001 | ||
| Elective | 155 (87.6) | 1,001 (96.8) | |
| Urgent/ER | 22 (12.4) | 33 (3.2) | |
| Operative approach | 0.001 | ||
| Open | 1 (0.6) | 2 (0.2) | |
| Laparoscopy | 168 (94.9) | 1,029 (99.5) | |
| Lap. converted to open | 8 (4.5) | 3 (0.3) | |
| Operative time (min) | 67 ± 38 | 54 ± 29 | 0.001 |
| Bleeding > 100 mL | 0.002 | ||
| Yes | 6 (3.4) | 5 (0.5) | |
| No | 171 (96.6) | 1,029 (99.5) | |
| GB perforation | 0.57 | ||
| Yes | 4 (2.3) | 23 (2.2) | |
| No | 173 (97.7) | 1,011 (97.8) | |
| Drain | 0.001 | ||
| Yes | 38 (21.5) | 109 (10.5) | |
| No | 139 (78.5) | 925 (89.5) | |
| Gangrenous GB | 0.001 | ||
| Yes | 10 (5.6) | 9 (0.8) | |
| No | 167 (94.4) | 1,025 (99.2) | |
| Wound infection | 0.68 | ||
| Yes | 0 (0) | 1 (0.1) | |
| No | 177 (100) | 4 (99.9) | |
| Collection | 0.87 | ||
| Yes | 1 (0.6) | 7 (0.7) | |
| No | 176 (99.4) | 1,027 (99.3) | |
| Stump leak | 0.70 | ||
| Yes | 1 (0.6) | 12 (1.2) | |
| No | 176 (99.4) | 1,022 (98.8) | |
| Re-visit to theater | 0.88 | ||
| Yes | 1 (0.6) | 5 (0.5) | |
| No | 176 (99.4) | 1,029 (99.5) | |
| Histopathology | 0.001 | ||
| Chronic cholecystitis | 151(85.3) | 981 (94.9) | |
| Others | 25 (14.1) | 53 (5.1) | |
| Length of stay (day) | 2.9 ± 1.6 | 2.3 ± 0.9 | 0.001 |
| 30-day readmission | 0.63 | ||
| Yes | 4 (2.3) | 18 (1.7) | |
| No | 173 (97.7) | 1,016 (98.3) | |
| 30-day mortality | 0 | 0 |
GBWT, gallbladder wall thickness; ASA score, American society of anesthesiologist score; WBC, white blood cell; HB, hemoglobin; SGOT, serum glutamic-oxaloacetic transaminase; AST, aspartate aminotransferase; ALT, alanine transaminase; INR, international normalized ratio; GB, gall bladder; CBD, common bile duct; ERCP, endoscopic retrograde choangiopancreatography.
Table 2
Univariate and multivariate analysis of variables associated with long hospitalization
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
|
|
|
||||
| p-valuea) | HR | 95% CI | p-valueb) | ||
| Age (yr) | 0.001 | ||||
| Duration of complain | 0.035 | ||||
| INR | 0.004 | ||||
| GBWT | 0.031 | 0.008 | |||
| GBWT (–) | 1 | ||||
| GBWT (+) | 1.97 | (1.19–3.25) | |||
| Surgery sitting | 0.001 | 0.001 | |||
| Elective | 1 | ||||
| Urgent/ER | 10.2 | (4.07–25.92) | |||
| Operative time | 0.04 | 0.001 | |||
| < 60 minutes | 1 | ||||
| > 60 minutes | 1.01 | (1.0–1.02) | |||
| Postoperative drain | 0.001 | 0.001 | |||
| No | 1 | ||||
| Yes | 11.3 | (6.40–20.0) | |||
| Postoperative collection | 0.04 | ||||
| No | |||||
| Yes | |||||




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download