Abstract
Backgrounds/Aims
Timing of resection for synchronous colorectal liver metastasis (CRLM) has been debated for decades. The aim of the present study was to assess the feasibility of simultaneous resection of CRLM in terms of major complications and develop a prediction model for safe resections.
Methods
A retrospective single-center study of synchronous, resectable CRLM, operated between 2013 and 2021 was conducted. Upper limit of 95% confidence interval (CI) of major complications (≥ grade IIIA) was set at 40% as the safety threshold. Logistic regression was used to determine predictors of morbidity. Prediction model was internally validated by bootstrap estimates, Harrell’s C-index, and correlation of predicted and observed estimates.
Results
Ninety-two patients were operated. Of them, 41.3% had rectal cancers. Major hepatectomy (≥ 4 segments) was performed for 25 patients (27.2%). Major complications occurred in 20 patients (21.7%, 95% CI: 13.8%–31.5%). Predictors of complications were the presence of comorbidities and major hepatectomy (area under the ROC curve: 0.692). Unacceptable level of morbidity (≥ 40%) was encountered in patients with comorbidities who underwent major hepatectomy.
Synchronous liver metastasis is present in 15% of colorectal cancer (CRC) patients. Nearly 50% of patients with colorectal liver metastasis (CRLM) have liver limited metastasis [1,2]. The approach to synchronously presenting resectable CRLM has been debated for decades without a consensus on timing or sequencing of liver and bowel operations. Prior series have examined various strategies, including simultaneous and staged resections with bowel or liver first approaches. Metanalysis of these studies has reported similar oncological outcomes with any of these strategies [3].
Simultaneous resections offer reduce overall hospital stay, costs, patient anxiety, and probability of progression between two operations of a staged approach. Despite the conceptual appeal, synchronous resections are not widely adopted. The hesitation is largely due to fear of additive major morbidity, referral after colorectal resections, and a strong surgical bias towards a particular approach of management. Arguably, the decision for simultaneous or staged resection should be based on the acceptable level of morbidity when cancer outcomes appear to be similar.
Previous retrospective studies have uniformly found higher complication rates with simultaneous resection [4-6]. The only randomized evidence comparing simultaneous and staged resection of synchronously presenting liver metastasis is the MetaSync trial [7]. The synchronous arm had a serious morbidity rate of 49% with better 2-year overall survival (OS) and disease-free survival (DFS). Another single-arm study investigating the feasibility of enrolling patients in a randomized trial of simultaneous or delayed resection also demonstrated a major morbidity rate of 41% [8]. These high morbidity rates from prospective studies reinforce apprehension of synchronous resections and cast doubt on the universal applicability of this approach and feasibility of future trials.
The present study aimed to assess complication rates of simultaneous resections, identify predictors of major morbidity, and develop a prediction model to estimate the safety of synchronous operations at our institute. The goal was to define a cohort where simultaneous resections would be safe for forthcoming prospective studies.
The prospectively maintained operative CRLM database of a tertiary referral cancer center was retrospectively reviewed. All patients with simultaneous operation for the CRLM and bowel cancer between February 2013 and December 2021 were included. Synchronous CRLM patients were electively planned for staged resections when they were considered unfit for simultaneous resection as decided by the joint multi-disciplinary team (MDT). Patients with a symptomatic, resectable bowel cancer underwent resection of the primary tumour first. Patients that were managed with thermal ablation for liver metastasis were not included. Those with CRLM requiring two-stage hepatectomy or preoperative volume augmentation for inadequate future liver remnant (FLR) and those with resections involving six or more liver segments were excluded.
CRCs included adenocarcinomas of the right colon (proximal to splenic flexure), left colon (splenic flexure till rectosigmoid junction), and the rectum (≤ 15 cm from the anal verge). Staging investigations for all CRCs included a colonoscopy, biopsy, contrast enhanced computed tomography (CT) of the chest, abdomen, and the pelvis, and magnetic resonance imaging for rectal cancers. Positron emission tomography was not performed for metastatic CRCs as a routine. CRLM was diagnosed on triphasic CT scans. Doubtful liver lesions were characterized using a combination of ultrasonography, magnetic resonance imaging and tissue sampling.
The approach to synchronously presenting CRLM was decided by the MDT. The preferred path was simultaneous resection unless the patient was unfit for combined procedure, planned extended liver resections (6 or more segments, biliary or vascular anastomosis), requirement of preoperative volume augmentation, or in the presence of symptomatic colonic primary requiring resection. For smaller (≤ 3 cm), deep-seated, limited (n = 1–3) liver lesions, thermal ablations were utilized on a case-to-case basis. Similarly, the decision for perioperative chemotherapy was individualized for resectable CRLM based on the need for preoperative therapy in advanced rectal cancers and the number, size, and distribution of liver metastasis. The Fong’s clinical risk score [9] and KRAS mutation status also contributed to the decision to deliver preoperative chemotherapy.
The sequence of operation during simultaneous resection relied on the complexity of individual operation. Usually, liver resection was performed prior to colorectal resection. Portal clamping was not routinely performed. Surgical approaches included open, laparoscopic, and robotic platforms. Rectal resections were usually performed by minimally invasive surgery (MIS) while colonic and liver resections were performed by open operations in the majority of patients. Major hepatectomy was defined as resection of 4 or more segments. It essentially included formal hemi-hepatectomies or extended hemi-hepatectomies. Wedge and non-anatomical resections in four segments were not considered as a major hepatectomy. For smaller metastasis, the preference was towards non-anatomical resections rather than inflow directed segmentectomies. FLR adequacy was defined as 30% without preoperative chemotherapy and 40% with prior chemotherapy. Resectability of liver metastasis in general was defined as the ability to achieve R0 with an adequate FLR.
Perioperative outcomes recorded were blood loss, hospital stay, 30-day postoperative complications and 90-day hospital readmissions. Complications were recorded on the Clavien-Dindo scale [10]. Grade IIIA or higher complications were regarded as serious or major morbidity. Liver specific complications recorded were post hepatectomy bile leaks, liver failure and hemorrhage per the International Study Group of Liver Surger (ISGLS) classification systems [11-13]. Colorectal anastomotic leak was defined clinically or based on radiological extraluminal contrast extravasation.
R0 resection for colorectal resections was considered for tumor-free margin > 1 mm while that for liver resections was considered when tumor was absent at the inked resection surface absence. OS was calculated from date of operation to the date of death. DFS was calculated from the date of operation to the date of recurrence. Liver recurrence-free survival (LRFS) was similarly derived from the date of surgery to the date of liver relapse.
Based on major complication rates in two prospective studies on synchronous resections [7,8], a 40% serious morbidity rate (≥ grade IIIA) was considered as the safety threshold. If the upper limit of the 95% confidence interval (CI) of major complications was over 40%, simultaneous resections were considered unsafe. In a previous institutional audit, 21% of liver resections had serious complications [14]. Assuming a 30% major morbidity rate with synchronous resections, at least 88 patients would be required to demonstrate the safety threshold with 95% confidence.
Data were recorded and analyzed using the statistical program for social sciences (IBM SPSS version 26; IBM Corp., Armonk, NY, USA). Continuous variables are presented as medians and ranges. Categorical variables are presented as numbers and proportions. The primary outcome of interest (serious complications) was reported with 95% confidence intervals. Median follow up was calculated by the reverse Kaplan-Meier method. Survivals analysis was performed using Kaplan-Meier curves.
Regression for binary categorical dependent variable was performed using logistic regression with odds ratio (OR) reported. Regression for time-to-event variables was carried out using Cox-regression analysis with hazard ratios reported. Independent variable selection for multivariate regression was done by backward elimination method using exit level alpha of 0.1. Log likelihood ratios were utilized for assessing model goodness-of-fit. A two-sided p-value of 0.05 was considered statistically significant.
A prediction model was created using independent variables reduced by backward elimination. Internal validation and correction for prediction with small datasets were performed using the principle of bootstrapping [15]. Bias corrected accelerated estimates and bootstrapped p-values were provided. Calibration or goodness-of-fit was assessed by comparing the observed number of events to the expected number derived from the prediction model. Discrimination ability of the model was tested using the Harrell’s c index. In the case of binary outcomes, the c-statistic is equivalent to the area under the ROC curve (AUC).
A signed informed consent was obtained from each patient during treatment, surgery and questionnaires. The study protocol followed the ethical standards of the institutional research committee and the 1964 Helsinki Declaration with its later amendments. Due to the retrospective nature of this study and the use of anonymized data, approval from the institutional review board was not mandated. All records were derived from the Electronic Medical records and all patients are a part of ongoing prospective audit that is approved by the IRB (IRB project number 1478).
The strengthening the reporting of observational studies guidelines were followed for study reporting. Transparent reporting of multivariable prediction model for individual prognosis or diagnosis and statistical analysis and methods in published literature were used for reporting statistical methodology and results.
Within the study period, 236 patients with CRLM were resected, including 141 who had a synchronous presentation. Forty-three patients underwent planned staged resection because of reasons listed in Fig. 1. Amongst 98 patients intended for simultaneous resection, the bowel resection was abandoned intraoperatively for six patients. Finally, 92 patients who underwent simultaneous bowel and liver resections were included for further analysis.
Median age of the cohort was 52.5 years. Thirty-eight (41.3%) patients had rectal cancers. Median number of liver metastases was two, ranging from one to eight with a median size of 3 cm. Bilobar liver metastases were present in 29 (31.5%) patients. Preoperative chemotherapy was assigned to 63 (68.5%) patients. Ten (10.9%) patients received targeted therapy (Table 1). Major hepatectomy (≥ 4 segments) was performed for 25 (27.2%) patients. Eight patients had rectal resections with major hepatectomy. Another eight patients had pelvic exenterations for advanced rectal cancers with minor hepatectomy.
Baseline characteristics of study subjects (n = 92)
Serious complications (≥ grade IIIA) were observed in 20 (21.7%, 95% CI: 13.8%–31.5%) patients. Thus, the safety threshold was not crossed by the cohort (Table 2). Specifically, bile leaks (any grade), post hepatectomy liver failure (≥ grade B) and anastomotic leak were present in 7 patients (7.6%), 12 patients (13.0%), and 7 patients (7.6%), respectively. Adjuvant therapy could be delivered to 83 (90.2%) patients. At a median follow-up of 42 months, 53 patients developed recurrences (Table 3). A majority of relapses were within the liver (39 patients). Three-year OS, DFS, and LRFS were 71.3%, 23.2%, and 51.3%, respectively (Fig. 2). Results of multivariate Cox regression analysis for factors predicting OS, DFS, and LRFS are shown in Supplementary Table 1.
Operative and pathological features of study subjects (n = 92)
Oncological outcomes (n = 92)
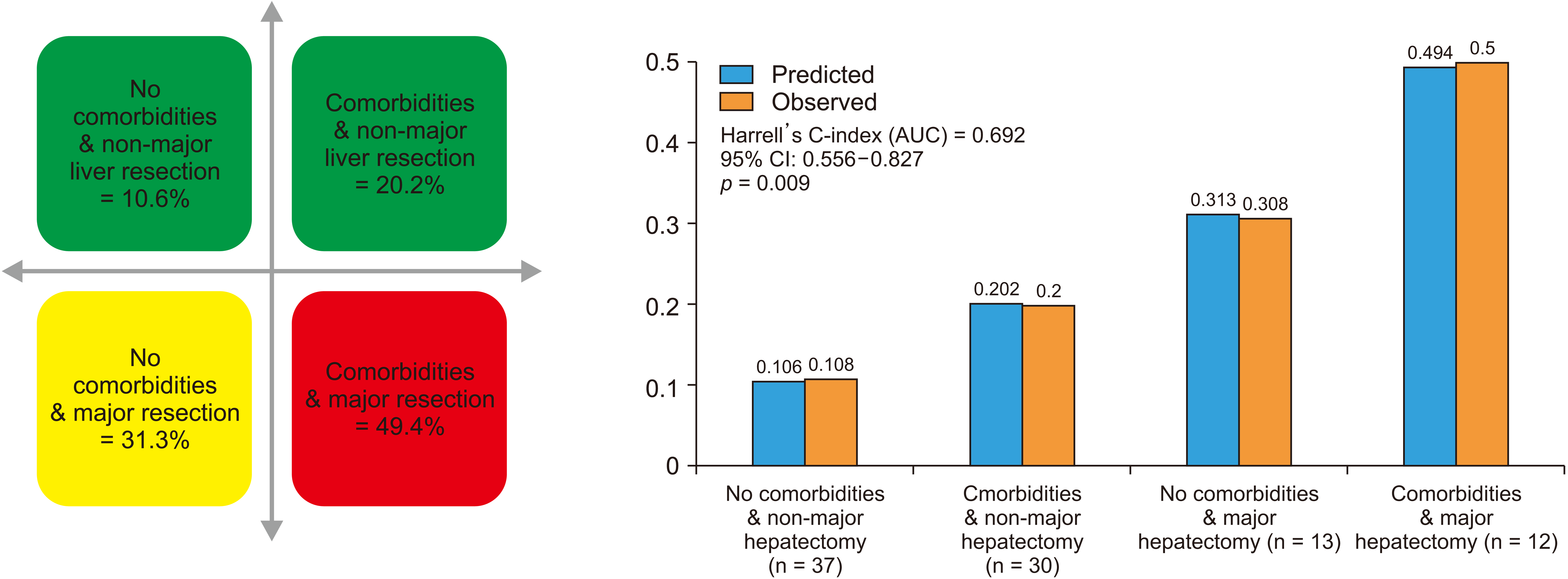
Prediction model for serious complications derived from logistic regression analysis and bootstrapping resulted in two variables (comorbidities and major hepatectomy) contributing to the model fit (Table 4). Only major hepatectomy had an independent predictive value (OR: 3.884; p = 0.016). The regression model had a better fit when comorbidities were incorporated (OR: 2.138; p = 0.145). Fig. 3 shows expected events (serious morbidity) derived from the prediction model for different permutations of comorbidities and extent of liver resection against observed events. Similarities between observed and expected event rates suggest adequate calibration, fit and internal validity of the model. The AUC of the derived mode was 0.692 (95% CI: 0.556–0.827; p = 0.009) (Supplementary Fig. 1). Thus, the model had a moderate discrimination ability. It could correctly predict the outcome for 70% of cases.
Logistic regression analysis for serious complications (≥ grade IIIA)
Based on the model, only patients with comorbidities undergoing major hepatectomy had a predicted probability of serious complications over 40% (49.4%), irrespective of colorectal operation.
In a largely unselected cohort of synchronous resectable CRLM, simultaneous resection appeared to be safe with a major complication rate of 21.7%. An internally validated prediction model estimated unacceptable level of complications (≥ 40%) in patients with comorbid conditions undergoing major liver resection, irrespective of colorectal operation.
Despite oncological equivalence, synchronous resections, especially combining rectal resections or major hepatectomies, are not routinely performed. The hesitation is largely due to high complication rates and a strong surgical bias towards a particular approach that could lead to difficulties in recruiting patients for simultaneous resection in prospective studies [7,8]. The philosophy of the MDT at our hospital preferred simultaneous resections irrespective of the primary resection or the extent of liver disease unless patient factors (comorbidities, performance status) or intraoperative complications precluded the combined resection. Less than 20% of patients eligible for simultaneous resection underwent staged resection at our hospital. This strengthened our results due to minimization of selection biases.
The fact that only previous comorbid illness and major hepatectomy influenced complications was not surprising since significant physiological changes were expected with both of these rather than a lower gastrointestinal resection. Our finding that colorectal resection did not influence complications was not an isolated result. Shubert et al. [16] have found similar results. Rectal resections with an anastomosis are usually considered as high-risk procedures to combine with liver operations [6]. Major hepatectomy with proctectomy is under-represented in most studies [6,7,17]. Over 40% of the present study cohort had rectal cancers and eight patients had major liver resections combined. Another eight patients had pelvic exenterations for advanced rectal cancers. Combining exenterations with liver resections appears to be safe based on the present series as well as PelvEx data [18].
The rate of serious morbidity was lower in our study than in the MetaSync trial despite similar distribution of colon and rectal cancers and similar distribution and extent of liver metastasis [7]. Some possible reasons might be younger patients with less comorbid conditions, adherence to enhanced recovery pathways [14], routine prophylactic diversion of low rectal anastomosis, exclusion of patients with highest risk of complications (extended hepatectomy, two-stage liver resection, inadequate FLR, and uncontrolled comorbidities) and limiting preoperative chemotherapy to three months. The growing acceptance of MIS for liver resections as well as advanced CRCs will further contribute to a reduction in morbidity [19].
Despite being the largest single institutional data on simultaneous resections with a cohort size larger than available prospective studies [7,8,17], the retrospective nature of our study imparts limitations that cannot be completely eliminated. Patients at highest risk of complications (i.e., uncontrolled comorbidities and inadequate FLR) were not planned for simultaneous resection. Thus, we cannot conclusively comment on outcomes of these patients. However, it is only logical to extrapolate from the prediction model that such patients are best treated in a staged manner.
The most significant drawback of this study that would limit its generalizability was that external validation of the prediction model was not performed. It might have a suboptimal discrimination ability. External validation of this tool is the logical next research question. We recognize that risk models that incorporate only two variables are an over-simplification of clinical situations. Nevertheless, increasing the number of variables could lead to over-fitting of the model and decrease its applicability outside the derived dataset when the event rate is low.
A comprehensive grading of comorbidities such as the Charlson comorbidity index would be more appropriate for risk stratification as opposed to the mere presence of comorbid illness. Similarly, the FLR and other parameters of preoperative liver functions are stronger predictors of postoperative outcomes than the number of segments resected. They should be incorporated in future studies. This study did not aim to compare oncological results of simultaneous resections against staged resections. In addition, many variables that could influence cancer outcomes were missing. Finally, over 80% of resections were performed via an open approach. In the present-day context, this is a limitation owing to higher short-term morbidity with an open operation.
Management of CRLM is surrounded by several uncertainties and deliberations. There are some vital questions regarding the use of perioperative chemotherapy in resectable CRLM and the timing of operation in synchronously presenting CRLM. With perioperative chemotherapy in CRLM being questioned by recent randomized trials [20,21], a larger proportion of patients are expected to be eligible for upfront simultaneous resections. The long-term aim should be comparing hard oncological outcomes by approaches that have not been successfully performed due to higher complication rates reported with simultaneous resections in prospective studies. Results from the present study can assist in the selection of patients for safe synchronous resections and refine eligibility for future prospective comparisons.
Simultaneous bowel and CRLM resection appear to be safe in experienced centers, including the combination of rectal and major liver resections in patients without comorbid conditions. Better selection criteria may allow prospective studies to be performed safely. However, the prediction model needs external validation.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.22-043.
REFERENCES
1. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. 2018; Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 18:78. DOI: 10.1186/s12885-017-3925-x. PMID: 29334918. PMCID: PMC5769309.

2. Patil PS, Saklani A, Gambhire P, Mehta S, Engineer R, De'Souza A, et al. 2017; Colorectal cancer in India: an audit from a tertiary center in a low prevalence area. Indian J Surg Oncol. 8:484–490. DOI: 10.1007/s13193-017-0655-0. PMID: 29203978. PMCID: PMC5705504.

3. Baltatzis M, Chan AK, Jegatheeswaran S, Mason JM, Siriwardena AK. 2016; Colorectal cancer with synchronous hepatic metastases: systematic review of reports comparing synchronous surgery with sequential bowel-first or liver-first approaches. Eur J Surg Oncol. 42:159–165. DOI: 10.1016/j.ejso.2015.11.002. PMID: 26733368.

4. Bogach J, Wang J, Griffiths C, Parpia S, Saskin R, Hallet J, et al. 2020; Simultaneous versus staged resection for synchronous colorectal liver metastases: a population-based cohort study. Int J Surg. 74:68–75. DOI: 10.1016/j.ijsu.2019.12.009. PMID: 31843676.

5. Snyder RA, Hao S, Irish W, Zervos EE, Tuttle-Newhall JE, Parikh AA. 2020; Thirty-day morbidity after simultaneous resection of colorectal cancer and colorectal liver metastasis: American College of Surgeons NSQIP analysis. J Am Coll Surg. 230:617–627.e9. DOI: 10.1016/j.jamcollsurg.2019.12.018. PMID: 32007534.

6. Tsilimigras DI, Sahara K, Hyer JM, Diaz A, Moris D, Bagante F, et al. 2021; Trends and outcomes of simultaneous versus staged resection of synchronous colorectal cancer and colorectal liver metastases. Surgery. 170:160–166. DOI: 10.1016/j.surg.2021.01.041. PMID: 33674128.

7. Boudjema K, Locher C, Sabbagh C, Ortega-Deballon P, Heyd B, Bachellier P, et al. 2021; Simultaneous versus delayed resection for initially resectable synchronous colorectal cancer liver metastases: a prospective, open-label, randomized, controlled trial. Ann Surg. 273:49–56. DOI: 10.1097/SLA.0000000000003848. PMID: 32209911.

8. Serrano PE, Parpia S, Karanicolas P, Gallinger S, Wei AC, Simunovic M, et al. 2022; Simultaneous resection for synchronous colorectal cancer liver metastases: a feasibility clinical trial. J Surg Oncol. 125:671–677. DOI: 10.1002/jso.26764. PMID: 34878649.

9. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. 1999; Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 230:309–318. discussion 318–321. DOI: 10.1097/00000658-199909000-00004. PMID: 10493478. PMCID: PMC1420876.

10. Dindo D, Demartines N, Clavien PA. 2004; Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 240:205–213. DOI: 10.1097/01.sla.0000133083.54934.ae. PMID: 15273542. PMCID: PMC1360123.
11. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. 2011; Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 149:680–688. DOI: 10.1016/j.surg.2010.12.002. PMID: 21316725.

12. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. 2011; Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 149:713–724. DOI: 10.1016/j.surg.2010.10.001. PMID: 21236455.

13. Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, et al. 2011; Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford). 13:528–535. DOI: 10.1111/j.1477-2574.2011.00319.x. PMID: 21762295. PMCID: PMC3163274.

14. Joshi R, Thomas MJ, Bansode R, Mitra A, Chaudhari V, Desouza A, et al. 2016; Hepatic resections and enhanced recovery pathway: an Indian encounter. Clin Nutr ESPEN. 12:E54. DOI: 10.1016/j.clnesp.2016.02.078.

15. Smith GC, Seaman SR, Wood AM, Royston P, White IR. 2014; Correcting for optimistic prediction in small data sets. Am J Epidemiol. 180:318–324. DOI: 10.1093/aje/kwu140. PMID: 24966219. PMCID: PMC4108045.

16. Shubert CR, Habermann EB, Bergquist JR, Thiels CA, Thomsen KM, Kremers WK, et al. 2015; A NSQIP review of major morbidity and mortality of synchronous liver resection for colorectal metastasis stratified by extent of liver resection and type of colorectal resection. J Gastrointest Surg. 19:1982–1994. DOI: 10.1007/s11605-015-2895-z. PMID: 26239515.

17. Chan AKC, Mason JM, Baltatzis M, Siriwardena AK. 2022; Management of colorectal cancer with synchronous liver metastases: an inception cohort study (CoSMIC). Ann Surg Oncol. 29:1939–1951. DOI: 10.1245/s10434-021-11017-7. PMID: 34716838.
18. PelvEx Collaborative. 2020; Simultaneous pelvic exenteration and liver resection for primary rectal cancer with synchronous liver metastases: results from the. Colorectal Dis. 22:1258–1262. DOI: 10.1111/codi.15064. PMID: 32294308.
19. Kazi M, Kumar NAN, Rohila J, Sukumar V, Engineer R, Ankathi S, et al. 2021; Minimally invasive versus open pelvic exenterations for rectal cancer: a comparative analysis of perioperative and 3-year oncological outcomes. BJS Open. 5:zrab074. DOI: 10.1093/bjsopen/zrab074. PMID: 34518872. PMCID: PMC8438253.

20. Kanemitsu Y, Shimizu Y, Mizusawa J, Inaba Y, Hamaguchi T, Shida D, et al. 2021; Hepatectomy followed by mFOLFOX6 versus hepatectomy alone for liver-only metastatic colorectal cancer (JCOG0603): a phase II or III randomized controlled trial. J Clin Oncol. 39:3789–3799. DOI: 10.1200/JCO.21.01032. PMID: 34520230.

21. Bridgewater JA, Pugh SA, Maishman T, Eminton Z, Mellor J, Whitehead A, et al. 2020; Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 21:398–411. DOI: 10.1016/S1470-2045(19)30798-3. PMID: 32014119.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download