Abstract
Hepatocellular carcinoma (HCC) accounts for most of the hepatic neoplasms and can also occur in ectopic liver tissue. We present a case of a 55-year-old male complaining of weight loss. The imaging studies reported a 2.9 cm nodule in the pancreatic body, with a neuroendocrine tumor diagnosis by cytology. A corpo-caudal pancreatectomy was performed. Pathology showed a well-differentiated HCC developed in ectopic liver tissue with free margins and no lymph node metastases. HCC presenting in ectopic liver tissue is rare. In this case, the preoperative study did not establish the diagnosis, warranting the need for suspicion of this neoplasm.
Hepatocellular carcinoma (HCC) accounts for most of the primary hepatic neoplasms and is the third leading cause of cancer-related deaths in the world [1,2]. HCC data in Portugal are scarce. According to GLOBOCAN 2018, the incidence of liver cancer in 2018 was 2.4%, with this disease being responsible for 4.7% of cancer-related deaths in the country [3].
HCC mainly affects patients with chronic liver disease (with known risk factors including chronic viral hepatitis B and C, alcoholic liver disease, nonalcoholic steatohepatitis, and hereditary hemochromatosis) and has an aggressive behavior with poor prognosis [2,4,5]. Historically, HCC is more prevalent in male and older patients, and approximately 70% to 80% of the patients present as a stage IV disease at diagnosis [2,5,6].
Ectopic liver tissue (ELT) incidence is approximately 0.23% to 0.7% and is mostly asymptomatic with rare complications, such as compression, pain, bleeding, or other diseases similar to the orthotopic liver [7,8]. ELT mostly occurs in the gallbladder, followed by hepatic ligaments and omentum. This entity may also be found in the spleen, retroperitoneum, pancreas, adrenal gland, portal vein, diaphragm, thorax, gastric serosa, testis, and umbilical vein [7,9-11]. All of the above are susceptible sites to HCC, which may occur in approximately 7% to 30% of cases of ELT [7,10].
We present a case of a 55-year-old male, physically active, without regular medication or relevant previous medical history apart from smoking (20 pack-years). Regarding family health history, his father (alive, 92-year-old) had a previous cancer diagnosis (prostate cancer and melanoma).
The patient complained of unexplained weight loss over three years, most pronounced within the last month (loss of 6.3% of body weight). He had no other B symptoms as well as digestive, urinary, or respiratory symptoms. There were no relevant findings on physical examination.
The upper gastrointestinal endoscopy showed antral gastritis and Helicobacter pylori infection. The lower gastrointestinal endoscopy revealed diverticular disease and several adenomatous polyps with low-grade dysplasia in the transverse, descending, and sigmoid colon.
An abdominal ultrasound revealed a 3.2 cm × 2.6 cm hypoechoic lesion with a regular contour in the upper retroperitoneum suspected to be an adenomegaly, so, a thoracoabdominal and pelvic computed tomography (CT) was requested (Fig. 1). The CT showed a 2.9 cm × 2.5 cm × 2.8 cm solid, well-defined nodular lesion, slightly hypervascular, inseparable from the superior border of the pancreatic body. The radiologist suspected a neuroendocrine tumor of the pancreas, with the hypothesis of gastrointestinal stromal tumor seeming unlikely. There were also several retroperitoneal lymph nodes, the largest with a short-axis diameter measurement of 1 cm, of uncertain pathological significance, and also a granuloma/calcified hamartoma in the right lung. No liver lesions were observed.
Fine-needle aspiration cytology guided by echoendoscopy was performed at another institution. Despite equivocal immunohistochemical study results, namely marked positivity for CAM 5.2 and for CD56 (100% of the cells), positivity for Ki67 (2% of the cells), and negativity for chromogranin and synaptophysin, the lesion was interpreted as a neuroendocrine tumor without atypia, supporting the radiologist’s first impression.
Subsequent blood tests showed that chromogranin A, gastrin, and insulin were within the normal range (Table 1).
A positron emission tomography (PET) 68Ga-DOTANOC scan (Fig. 2) only identified a lesion of uncertain etiology located in the upper surface of the body of the pancreas with discrete to moderate uptake, hypothesizing that it would be a tumor with low expression of somatostatin receptors. There was no uptake in the right upper lung lesion.
After discussion in a multidisciplinary team meeting, an open corpo-caudal pancreatectomy was performed.
Pathology results (Fig. 3) revealed a well-differentiated HCC developed in ectopic hepatic tissue in the pancreas, with free resection margins and no lymph node metastases.
The histochemical/immunohistochemical study (Fig. 4) revealed: bile production (Hall’s histochemical method); tumor cell positivity for cytokeratins 8/18 and polyclonal carcinoembryonic antigen (CEA) (canalicular pattern); expression of CD34 demonstrating endothelialization of sinusoidal vascular spaces; multifocal positivity for HepPar-1 in tumor cells; tumor cells negativity for cytokeratin 7, Glipican-3, S100 protein, synaptophysin, and chromogranin A.
At the multidisciplinary team meeting, the patient was proposed a surveillance program.
Currently, at 49 months of follow-up, the patient has no signs of recurrence.
Informed patient consent was obtained for case publication.
HCC presenting in ELT is rare, although more frequent than in the orthotopic liver. In addition, it is less associated with risk factors characteristic of HCC (such as chronic viral hepatitis B and C and cirrhosis). Several theories have been proposed to explain the embryogenesis of ELT. It may occur due to early or late developmental anomalies from aberrant hepatic tissue migration or stem from pluripotent endoderm. ELT has an increased propensity for malignant transformation in comparison to the orthotopic liver due to abnormal functional architecture, namely arterial supply or venous and biliary drainage, leading to longer exposure to carcinogenic factors, which facilitates carcinogenesis [7-10,12-15]. This impaired transfer of blood and bile may also be the explanation for the better prognosis of HCC originating from ELT, as opposed to HCC in the orthotopic liver [11].
The diagnosis of HCC relies on both morphology and positivity for lineage-defining immunohistochemical stains such as HepPar-1. Additional immunohistochemical studies can be performed to further confirm the diagnosis, with Arginase-1 (less sensitive than HepPar-1 in well-differentiated HCC), CEA (highlighting a characteristic “canalicular pattern”), and CD34 (showing “endothelization of sinusoids”) being the most widely used [16]. This case of HCC in the pancreas belongs to the wider, recently introduced diagnostic category of pancreatic hepatoid carcinomas in accordance with the new edition of the World Health Organization book of digestive system tumors [16]. Three theories have been postulated to elucidate its pathogenesis: the first theory presumes that hepatic and pancreatic cells share a common stem progenitor deriving from the foregut endoderm. According to this theory, liver-specific genes might be reactivated in the pancreatic stem cells during oncogenesis. Another reasonable hypothesis is that differentiated pancreatic cells may have the ability to directly transdifferentiate into hepatocytes. Lastly, many hepatoid carcinomas may just represent HCC developing within the intrapancreatic ELT, which, as discussed above, is prone to oncogenesis [17]. For our case, we favored the last hypothesis for two main reasons. First, as opposed to the classic infiltrative pattern of primary pancreatic ductal adenocarcinomas, our neoplasm displayed well-defined rounded margins, reproducing the classic pattern of expansive growth we would expect in any other primary HCC of the liver. Second, since the surrounding parenchyma was devoid of any residual conventional ductal pancreatic adenocarcinoma, we find the theories of stem cell reprogramming and differentiated pancreatic cells transdifferentiation less probable.
To the best of our knowledge, there are six published cases of HCC in ELT in the pancreas [8,11,15,18-20], diagnosed in three male and three female with a mean age of 57-year-old (44–71 years). None of the patients presented significant comorbidities or risk factors for HCC, apart from an occult hepatitis B virus (HBV) infection in the case reported by Li et al. [15]. Half of the patients were asymptomatic and the pancreatic nodules were incidentally discovered in routine imaging exams [15,18,19], whereas the other three complained of abdominal pain or discomfort [8,11,20]. The mean diameter of the pancreatic nodules was 4.6 cm, which were mainly located in the tail of the pancreas (n = 5); the remaining patient had two masses in the body and head of the pancreas [15]. There are no pathognomonic imaging characteristics that raise the suspicion of HCC in ELT, as the typical radiological appearance of the HCC in the orthotopic liver is less distinctive in the ELT, namely a less noticeable enhancement of the HCC in the arterial phase and a less apparent rapid “wash-out” effect in the portal phase. These features often point to other diagnoses, as in our case or the ones described by Cardona et al. [8], Braun et al. [11], and Kubota et al. [19], where radiologically the tumor was interpreted as a pancreatic neuroendocrine tumor. Only two of the patients were submitted to fine-needle aspiration cytology, but the diagnosis of HCC was not suggested [8,18]. Due to the lack of guidelines, this entity is treated as HCC in the liver, with an excellent prognosis after surgical excision [7-9,11]. There are also reports of complete cure after surgery (enucleation or distal pancreatectomy) in cases of HCC in ELT in the pancreas [11,15,18,19]. However, Kelly found positive resection margins after distal pancreatectomy, along with extensive lymphovascular invasion leading to a completion pancreatectomy followed by adjuvant chemotherapy with gemcitabine and carboplatin [20]. Ultimately this patient was diagnosed with liver metastases at 22 months of follow-up and was alive at the time of that publication.
In these case reports and also in our patient, the preoperative study did not establish the diagnosis. The authors warn of the need for suspicion of these neoplasms that present no specific sign or symptom and are difficult to diagnose preoperatively. Nevertheless, despite patients with ELT being at higher risk for HCC, it is important to stress the favorable prognosis in comparison to HCC in the orthotopic liver.
REFERENCES
1. Forman D, Ferlay J. Stewart BW, Wild CP, editors. 2014. The global and regional burden of cancer. World cancer report 2014. International Agency for Research on Cancer;Lyon: p. 16–53.
2. Zhuo Y, Chen Q, Chhatwal J. Hoshida Y, editor. 2019. Changing epidemiology of hepatocellular carcinoma and role of surveillance. Hepatocellular carcinoma: translational precision medicine approaches. Humana Press;Cham: p. 53–67.

3. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Population Fact Sheets - Portugal [Internet]. Available from: https://gco.iarc.fr/today/fact-sheets-populations. Lyon: Cancer Today/IARC;2019. cited 2020 Oct 8.
4. European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. 2012; EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 56:908–943. Erratum in: J Hepatol 2012;56:1430. DOI: 10.1016/j.jhep.2012.03.006.
5. Hartke J, Johnson M, Ghabril M. 2017; The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 34:153–159. DOI: 10.1053/j.semdp.2016.12.011. PMID: 28108047.

6. Lee HW, Cho KJ, Park JY. 2020; Current status and future direction of immunotherapy in hepatocellular carcinoma: what do the data suggest? Immune Netw. 20:e11. DOI: 10.4110/in.2020.20.e11. PMID: 32158599. PMCID: PMC7049588.

7. Lee JY, Kim KH, Kang MS, Kim KH. 2015; Ectopic hepatocellular carcinoma arising from the peritoneum in a patient with a history of oropharyngeal cancer: a case report. Case Rep Oncol. 8:456–460. DOI: 10.1159/000441020. PMID: 26600779. PMCID: PMC4649744.

8. Cardona D, Grobmyer S, Crawford JM, Liu C. 2007; Hepatocellular carcinoma arising from ectopic liver tissue in the pancreas. Virchows Arch. 450:225–229. DOI: 10.1007/s00428-006-0353-8. PMID: 17216188.

9. Kanzaki R, Yamada T, Gotoh K, Takahashi H, Ohigashi H, Ishikawa O. 2010; Ectopic hepatocellular carcinoma arising in the left triangular ligament of the liver. Case Rep Gastroenterol. 4:138–143. DOI: 10.1159/000314042. PMID: 20805935. PMCID: PMC2929406.

10. Adachi Y, Hayashi H, Yusa T, Takematsu T, Matsumura K, Higashi T, et al. 2020; Ectopic hepatocellular carcinoma mimicking a retroperitoneal tumor: a case report. World J Gastroenterol. 26:2268–2275. DOI: 10.3748/wjg.v26.i18.2268. PMID: 32476791. PMCID: PMC7235206.

11. Braun M, Kuncman W, Teresiński L, Kupnicki P, Jesionek-Kupnicka D, Kordek R. 2017; Pure hepatocellular carcinoma originates from an ectopic liver nodule located in the pancreas. Contemp Oncol (Pozn). 21:311–314. DOI: 10.5114/wo.2017.72403. PMID: 29416439. PMCID: PMC5799705.

12. Matsuyama M, Sugiura S, Kakita A, Sato Y, Kuroda M. 2011; Hepatocellular carcinoma arising from ectopic liver tissue in the spleen producing insulin-like growth factor II. Pathol Res Pract. 207:124–126. DOI: 10.1016/j.prp.2010.09.003. PMID: 20943327.

13. Caygill C, Gatenby P. 2004; Ectopic liver and hepatocarcinogenesis. Eur J Gastroenterol Hepatol. 16:727–729. DOI: 10.1097/01.meg.0000131037.92864.df. PMID: 15256972.

14. Mani VR, Farooq MS, Soni U, Kalabin A, Rajabalan AS, Ahmed L. 2016; Case report of ectopic liver on gallbladder serosa with a brief review of the literature. Case Rep Surg. 2016:7273801. DOI: 10.1155/2016/7273801. PMID: 27803835. PMCID: PMC5075603.

15. Li Z, Wu X, Wen T, Li C, Peng W. 2017; Multiple ectopic hepatocellular carcinomas in the pancreas: a case report. Medicine (Baltimore). 96:e6747. DOI: 10.1097/MD.0000000000006747. PMID: 28746170. PMCID: PMC5627796.
16. WHO Classification of Tumours Editorial Board. 2019. Digestive system tumours: WHO classification of tumours, Volume 1. 5th ed. International Agency for Research on Cancer;Lyon:
17. Zeng SX, Tan SW, Fong CTH, Liang Q, Zhao BL, Liu K, et al. 2020; Hepatoid carcinoma of the pancreas: a case report and review of the literature. World J Clin Cases. 8:1116–1128. DOI: 10.12998/wjcc.v8.i6.1116. PMID: 32258082. PMCID: PMC7103969.

18. Steen S, Wolin E, Geller SA, Colquhoun S. 2013; Primary hepatocellular carcinoma ("hepatoid" carcinoma) of the pancreas: a case report and review of the literature. Clin Case Rep. 1:66–71. DOI: 10.1002/ccr3.26. PMID: 25356215. PMCID: PMC4184752.

19. Kubota K, Kita J, Rokkaku K, Iwasaki Y, Sawada T, Imura J, et al. 2007; Ectopic hepatocellular carcinoma arising from pancreas: a case report and review of the literature. World J Gastroenterol. 13:4270–4273. DOI: 10.3748/wjg.v13.i31.4270. PMID: 17696261. PMCID: PMC4250631.

20. Kelly PJ, Spence R, Dasari BV, Burt AD, Taylor M, Loughrey MB. 2012; Primary hepatocellular carcinoma of the pancreas: a case report and review of the heterogeneous group of pancreatic hepatoid carcinomas. Histopathology. 60:1012–1015. DOI: 10.1111/j.1365-2559.2011.04129.x. PMID: 22320681.

Fig. 1
Thoracoabdominal and pelvic computed tomography (CT) showing a 2.9 cm × 2.5 cm × 2.8 cm solid well-circumscribed nodule inseparable from the superior border of the pancreatic body to the left of the celiac trunk, apparently irrigated by a branch of the splenic artery. The lesion is slightly hypervascular and does not have direct contact with the gastric wall. Due to its characteristics, it was considered more likely to be a neuroendocrine tumor of the pancreas, requiring characterization by biopsy, with the hypothesis of a gastrointestinal stromal tumor being unlikely. The remaining pancreatic gland presents a homogeneous texture of the parenchyma, without anomalous dilation of the Wirsung duct. The liver has a normal morphology and dimensions, presenting a homogeneous parenchymal texture. Along the retroperitoneum and less prominently in the mesentery, several lymph nodes may be observed, the largest an interaortocaval lymph node with a short-axis diameter measurement of 1 cm, of uncertain pathological significance. Coronal view (A) and axial view: arterial phase (B) and portal phase (C).
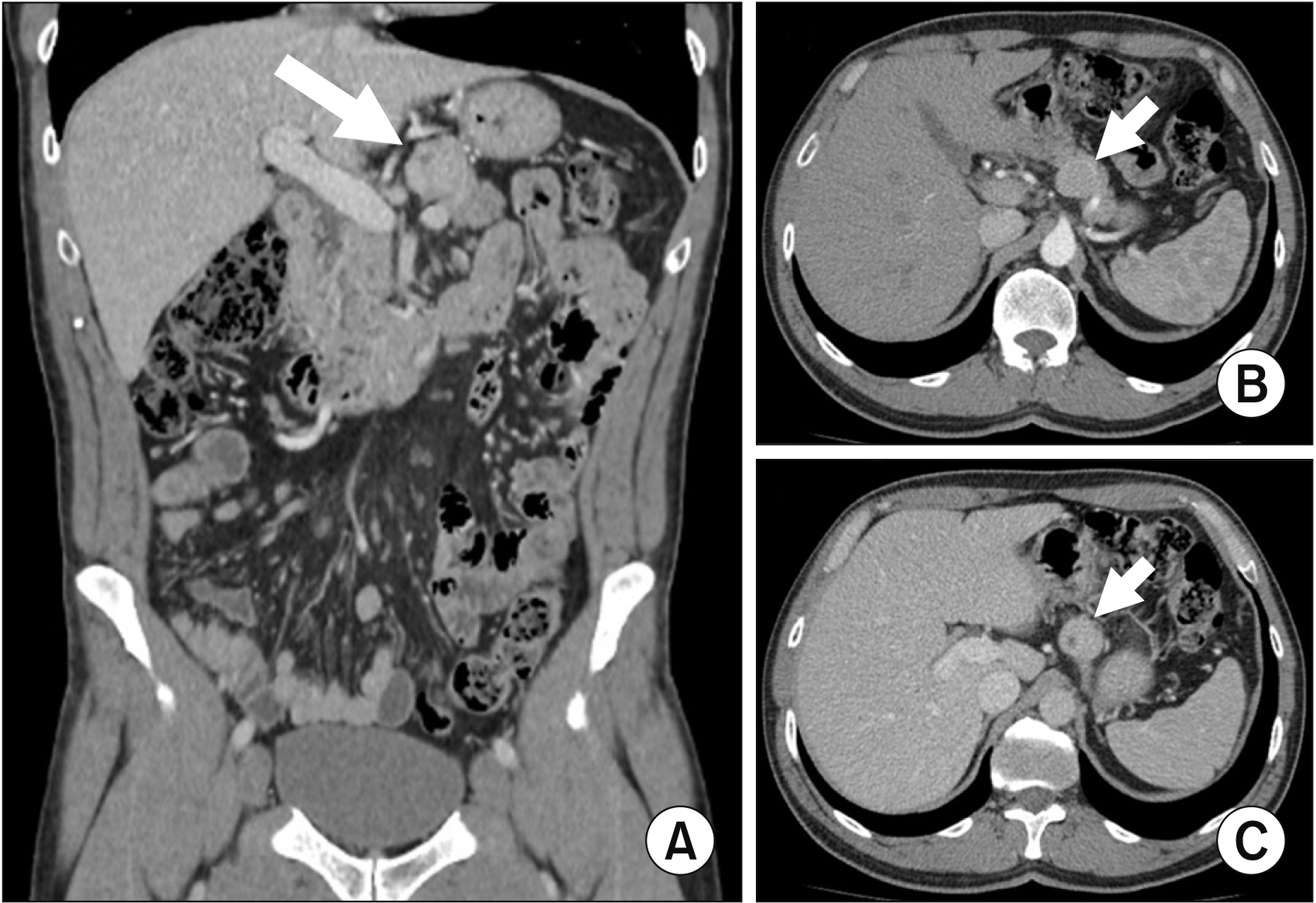
Fig. 2
Positron emission tomography (PET) 68Ga-DOTANOC scan. Discrete to moderate 68Ga-DOTANOC uptake (SUVmax = 5.8) in a lesion of uncertain etiology located in the upper surface of the body of the pancreas body (arrow): a tumor with low expression of somatostatin receptors? In the remaining study, no other foci of anomalous or significantly increased uptake were observed. Absence of 68Ga-DOTANOC uptake in the right upper lobe lung lesion.
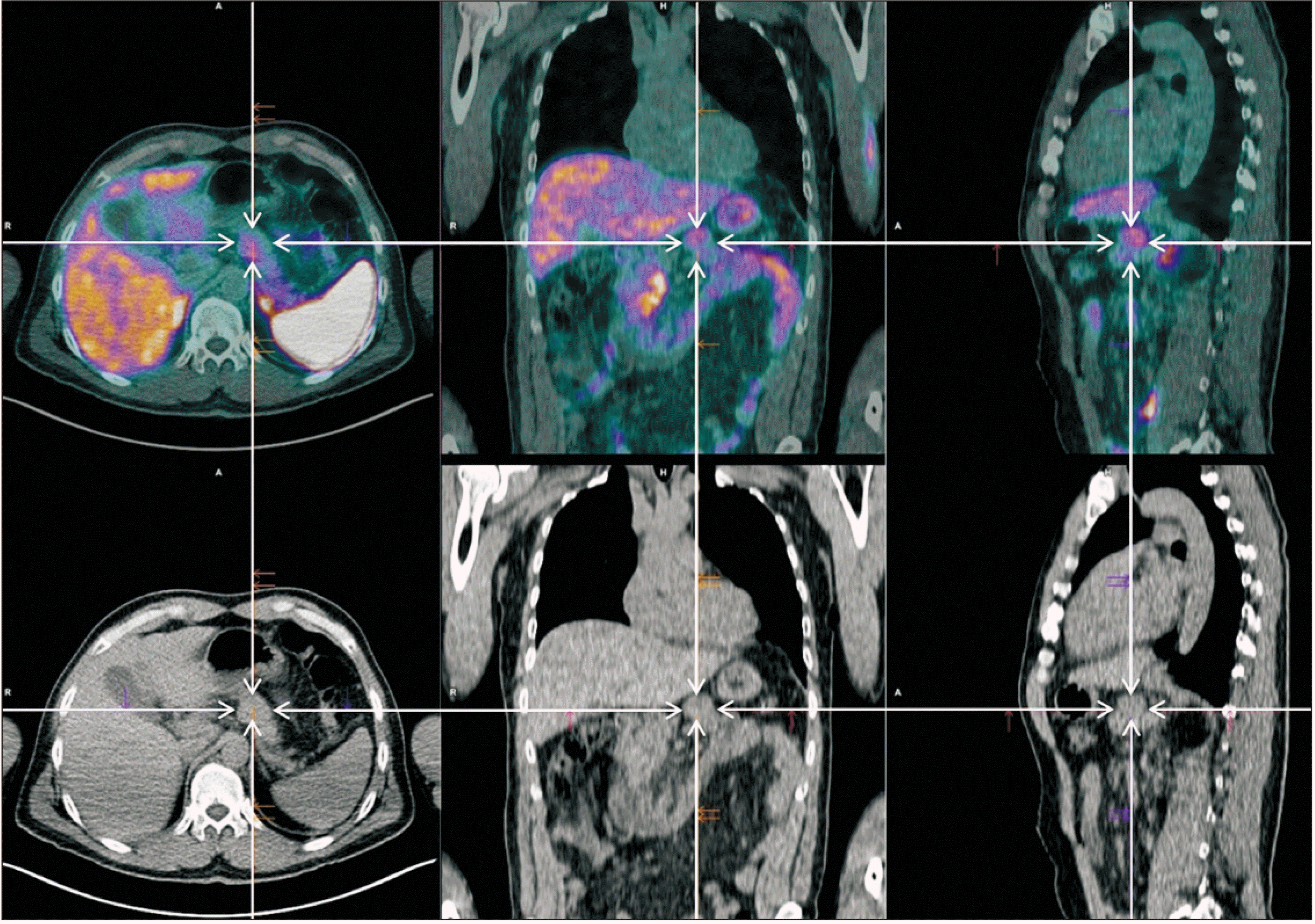
Fig. 3
Gross view of the formalin-fixed surgical specimen. (A) Pancreatic tail with a solid and circumscribed tumor. (B) Detail of the limit of the pancreatic parenchyma and the tumor.
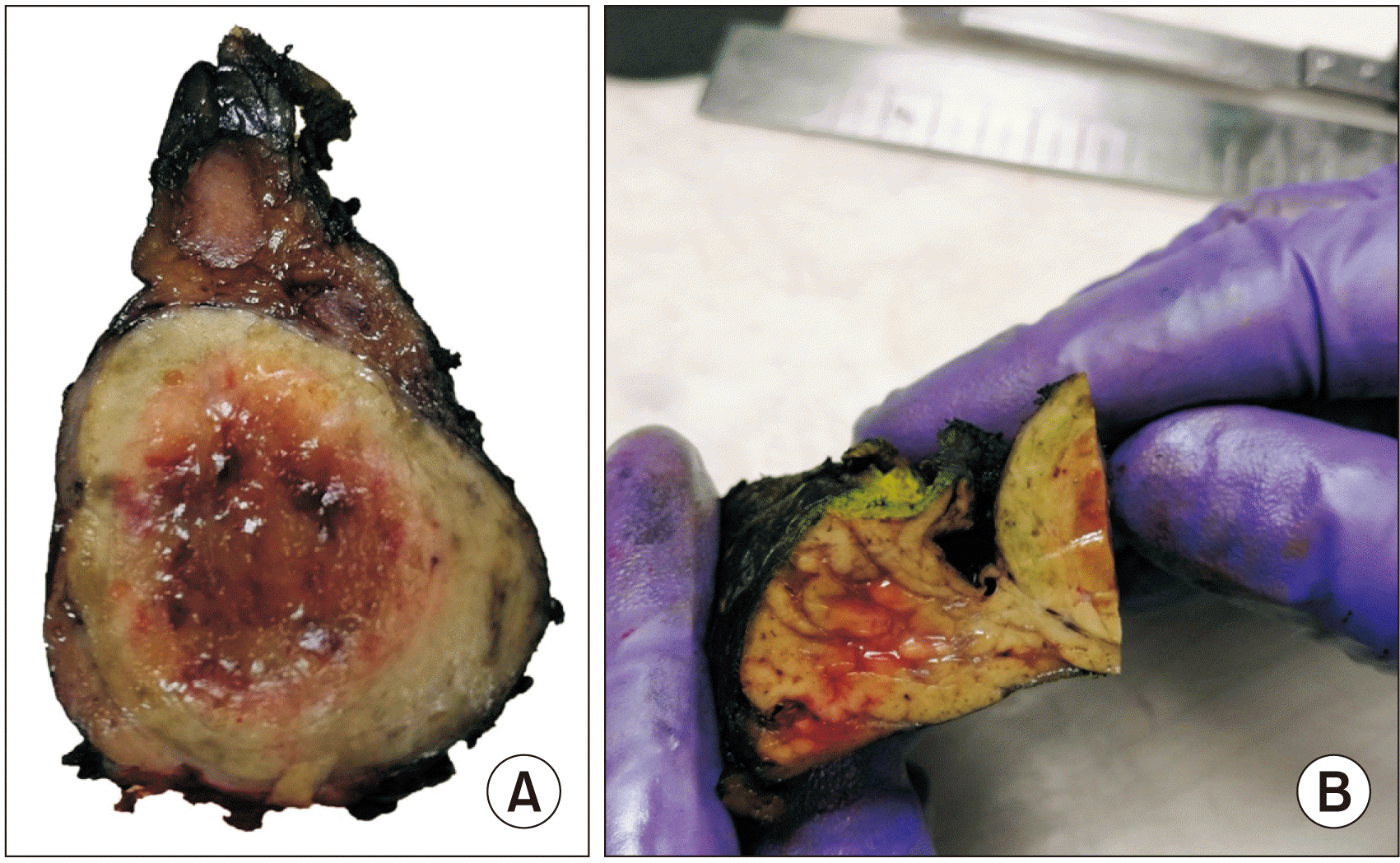
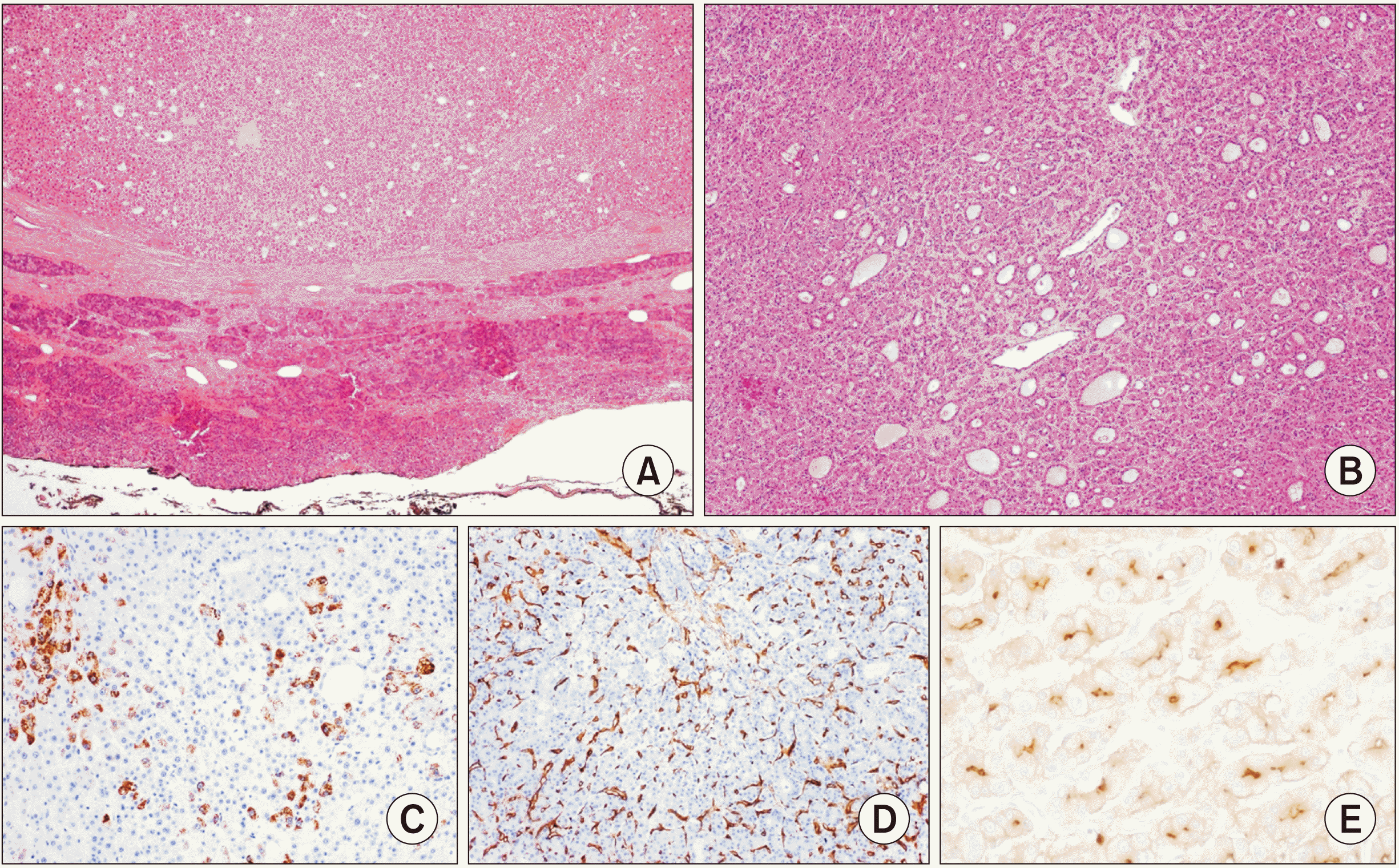
Fig. 4
Photomicrographs of the tumor. (A) Solid neoformation, predominantly wellcircumscribed and surrounded by a thin fibrous capsule (H&E, 40×). (B) Coexistence of two distinct microscopic patterns (pseudoglandular and trabecular): a common finding in hepatocellular carcinomas (H&E, 40×). (C) A positive reaction for HepPar1 confirms the hepatocellular nature of the lesion (DAB, 100×). (D) A positive reaction for CD34 in the hepatic sinusoids is a sign of “capillarization,” typical of hepatocellular carcinomas (DAB, 100×). (E) Positivity for polyclonal carcinoembryonic antigen (CEA) (canalicular pattern) further supports the hepatocellular differentiation of the neoplasm (DAB, 400×).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download