INTRODUCTION
Despite small bowel comprises 75% of the gastrointestinal tract and 90% of the mucosal surface, small bowel neoplasms are uncommon; fewer than 5% of gastrointestinal cancers [
1]. Primary adenocarcinoma of the duodenum represents only 0.5% of all intestinal neoplasms and constitutes approximately 50% of malignant lesions of the small intestine. According to Korea's annual report on cancer statistics from 2018, 976 cases each year or 0.4% of all cancer diagnoses have been documented [
2].
Surgery is the most effective treatment. Pancreatoduodenectomies are commonly performed on duodenal cancer. However, 25% of primary duodenal adenocarcinoma (DA) is unresectable because of advanced invasion [
3]. In which case, chemotherapy and radiotherapy can be used as alternative treatment. But there is no standardized form of chemotherapy due to low incidence.
Neoadjuvant chemotherapy can be a salvage therapy for unresectable duodenal cancer. Neoadjuvant chemotherapy is commonly used in locally advanced breast cancer which shrinks downstages tumor lesion, allowing smaller resection. When it comes to duodenal cancer, neoadjuvant chemotherapy showed positive results [
4,
5], but the few published cases available limits our understanding on the current scientific evidence. Herein, we are presenting the case of a male patient with stage 3 DA who received neoadjuvant chemotherapy with a positive result.
Go to :

CASE
Case presentation
In January 2016, a 51-year-old male patient initially visited the hospital for duodenal cancer bleeding impression found from esophagogastroduodenoscopy (EGD), conducted due to four times of massive hematochezia three days ago. He had no medical, surgical and medication history. He had no known family history of malignancy. He smoked for 25 pack-years and was not an excessive alcohol drinker.
An irregularly shaped ulcerofungating mass with nodular surface at duodenal 2nd portion was found in EGD and four times of biopsy was done along with electrical coagulation of the bleeding site. The biopsy revealed as DA. Laboratory results revealed the following: carbohydrate antigen (CA) 19-9, 1,353.0 U/mL; carcinoembryonic antigen (CEA) 3.66 ng/mL; hemoglobin 8.1 g/dL; aspartate transaminase, 17 U/L; alanine transaminase, 12 U/L; alkaline phosphatase, 64 U/L; total bilirubin, 0.6 mg/dL. Computed tomography (CT) (
Fig. 1A,
1B), magnetic resonance imaging (MRI) (
Fig. 1C), and positron emission tomography-computed tomography (PET-CT) (
Fig. 1D) identified a duodenal cancer arising from duodenal 1st and 2nd portion with regional metastatic lymph nodes (LNs) and vascular invasion, abutting aberrant right hepatic artery (aRHA), main portal vein (PV), and superior mesenteric vein (SMV). Since no distant metastasis lesion was found, the duodenal cancer was determined as stage III.
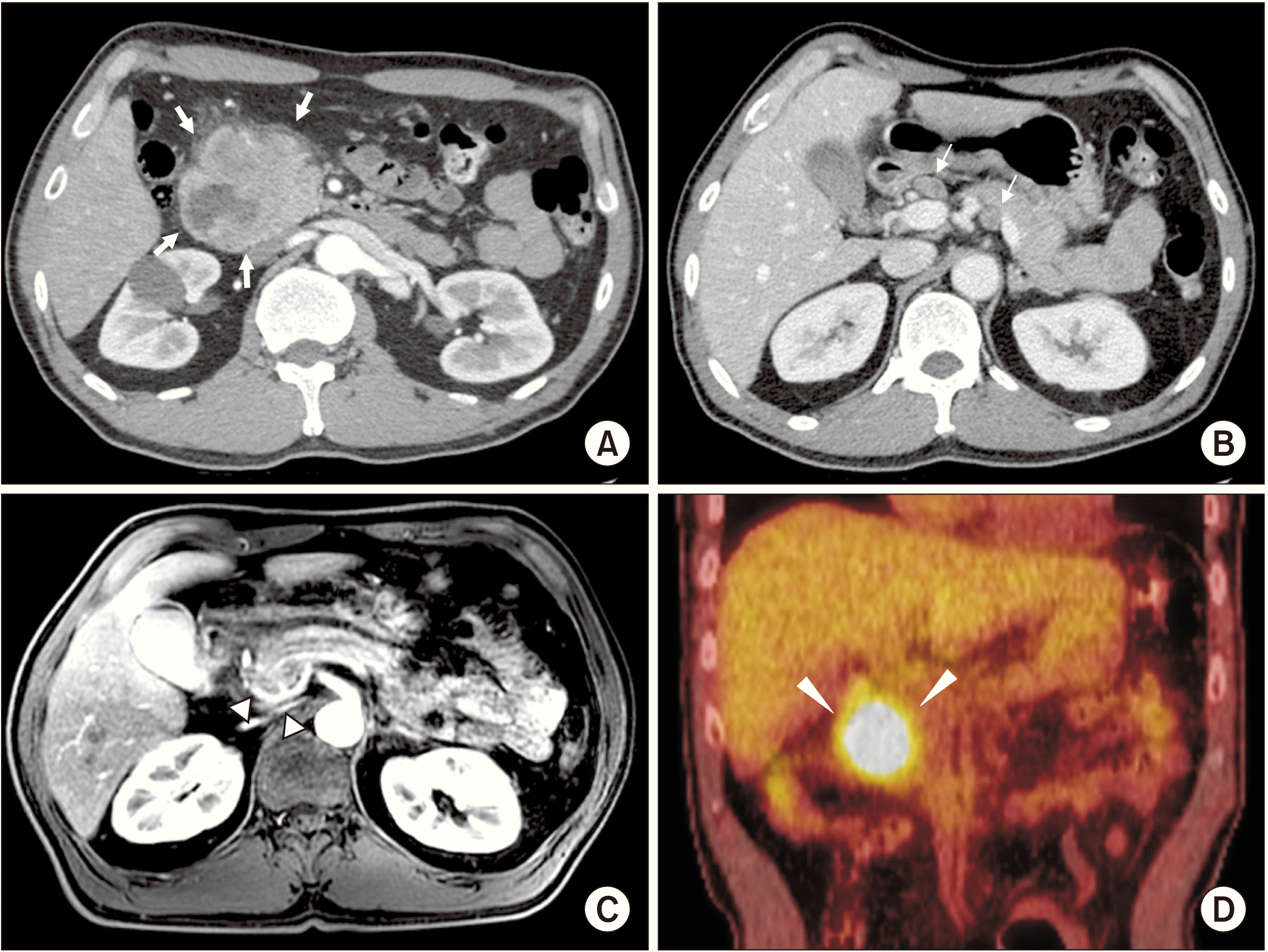 | Fig. 1Preoperative findings. Computed tomography scan shows duodenal tumor arising from the duodenal 1st and 2nd portions, with direct invasion of pancreas head (white arrows) (A). Metastatic lymph nodes in hepatoduodenal ligament, around celiac axis, and in gastric pyloric areas are presented (white arrows) (B). (C) Magnetic resonance imaging shows peritumoral infiltration abutting aberrant right hepatic artery from superior mesenteric artery, and main portal vein (white arrowheads). (D) Positron emission tomography-computed tomography shows hypermetabolic lesion in the duodenal 1st and 2nd portions (white arrowheads). 
|
The patient underwent chemotherapy with FOLFIRI (5-fluorouracil, leucovorin, irinotecan) (180 mg/m
2 of irinotecan, 200 mg/m
2 of leucovorin, and a total of 1,600 mg/m
2 of fluorouracil). After eight times of FOLFIRI, follow-up CT showed decreased tumor size to 2.1 cm from 5.7 cm, without significant change of probable metastatic LNs size. However, after the following eight administrations of FOLFIRI, no notable improvement was observed, despite slightly enlarged probably metastatic LNs in the hepatoduodenal ligament and around the celiac axis. Also, both tumor marker CA19-9 and CEA elevated to 30,56 U/mL and 5.80 ng/mL, respectively. Moreover, the patient appealed intolerability and preferred regimen which could be received during outpatient clinic rather than during admission. Thus, chemotherapy regimen was alternated to XELOX (capecitabine, oxaliplatine). In January 2017, finished four times of XELOX, follow-up CT scan showed decreased primary duodenal tumor and decreased seize of probable metastatic LNs. Following PET-CT showed decreased fluorodeoxyglucose (FDG) uptake of duodenal primary tumor and no other significant abnormal FDG uptake of suspicious LNs or distant metastasis. CA19-9 was also decreased to 32.6 U/mL (
Fig. 2). Thus, the patient’s duodenal tumor was expected to be capable for en-bloc resection.
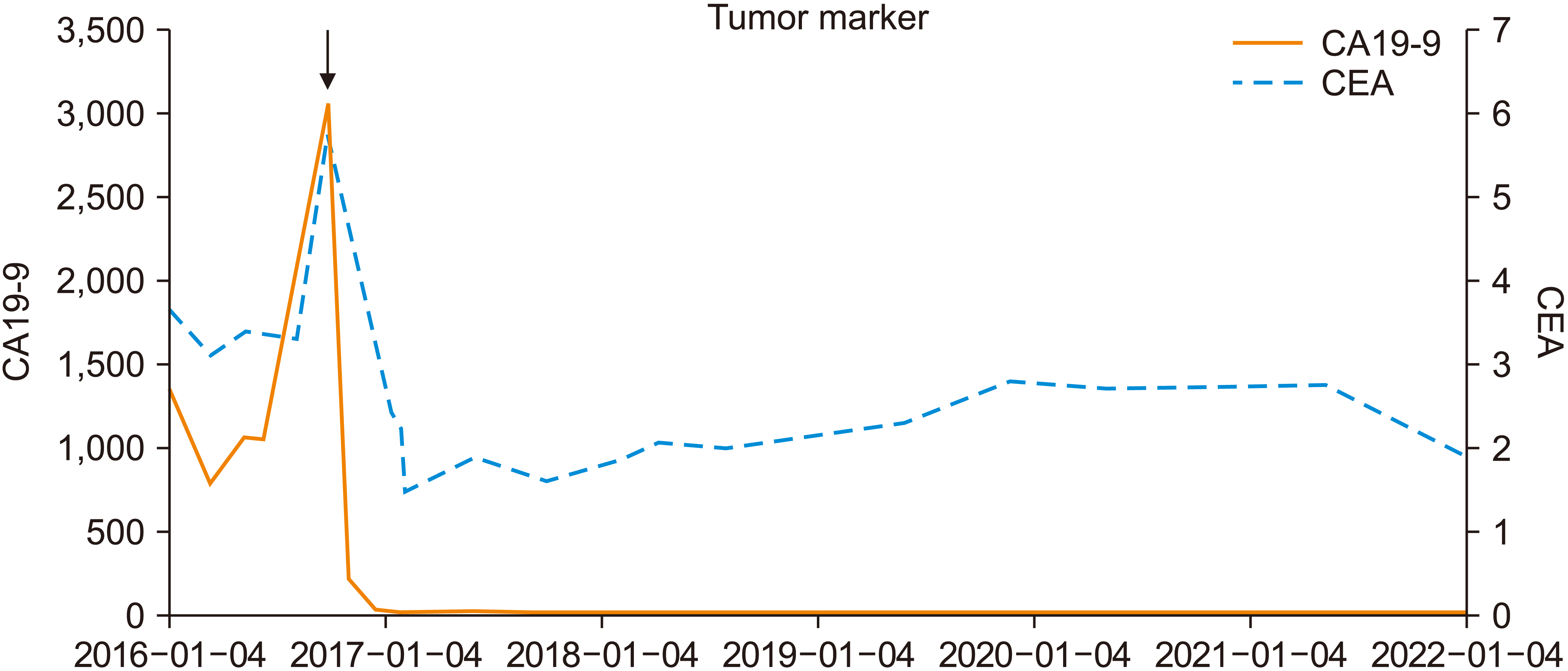 | Fig. 2
Change of tumor marker (CA19-9, CEA) during chemotherapy and after surgery. Both tumor markers decreased
dramatically after changing chemotherapy regimen to XELOX from FOLFIRI. CA, carbohydrate antigen; CEA, carcinoembryonic antigen; FOLFIRI, 5-fluorouracil, leucovorin, irinotecan; XELOX, capecitabine, oxaliplatine.

|
Operative finding
The patient underwent laparoscopic pylorus preserving total pancreatoduodenectomy (PPPD) on February 2nd, 2017. No peritoneal seeding or systemic metastasis was noted. Due to previous tumor shrinkage effect, en-bloc resection was done while the tumor was attached to colonic mesentery. Divisions of mid-colic and right colic artery was found in resected mesentery, yet the colon perfusion was normal. The aRHA arising from the origin of superior mesenteric artery (SMA) was confirmed. Although the 3rd and 4th portion of duodenum was closely attached to the origin of SMA, where the aRHA was branching, individual small vessels were carefully controlled and the lateral margin of SMA was secured. Uncinate process of pancreas was resected while securing the lateral margin of SMA. Infrapyloric, SMV, and paraaortic LNs were resected. Pancreatojejunostomy, hepaticojejunostomy, and duodenojejunostomy were done.
Pathological examination
Frozen biopsy of resected SMV, infrapyloric, and paraaortic LNs during operation were reported as free from tumor. There was no definite mass like lesion grossly on sections of duodenum and pancreas. Ampulla of Vater of the pancreas showed status of post chemotherapy with rare residual cancer cells in the peripancreatic tissue. Lymphovascular or perineural invasions were not identified, and all resection margins were free of carcinoma. Gallbladder, hepatoduodenal ligament, and four retrieved regional LNs were also free of carcinoma. It was found that primary tumor was perforating the visceral peritoneum, not directly invading to adjacent organ and major vascular structures, suggesting pathological stage T4N0M0 (Stage II), according to the American Joint Committee on Cancer TNM staging classification [
6].
Postoperative course and follow-up
Amylase and lipase levels of the peritoneal fluid peaked to 2,892 U/L and >6,900 U/L, respectively, on postoperative day 1. However, it decreased to 24 U/L and 61 U/L, respectively, on postoperative day 5. He had fever up to 38℃ until postoperative day 2 yet stabled after and discharged on day 8.
Follow-up evaluation was done every four months. Follow-up CT scan in May 2017 showed focal low attenuated lesion around gallbladder but was revealed with no evidence of recurrence or metastasis in liver MRI, thus postoperative chemotherapy was not planned. In January 2018, a newly developed lesion in the liver was shown yet no evidence of recurrence or metastasis was revealed in following liver MRI. Four to twelve month follow-up CT scan was done, and no evidence of tumor recurrence was demonstrated. In October 2021, the patient had epigastric pain and EGD and biopsy identified benign duodenal ulcer. The patient had stable CA19-9, CEA level (
Fig. 2) and was determined as completely cured in February 2022.
Surgical experience of surgical resection after neoadjuvant chemotherapy on duodenal cancer
Since 2011, a total of five patients had undergone neoadjuvant chemotherapy and their clinical characteristics are demonstrated in
Table 1. With a patient age range of 51 to 81 years and a median of 65 years, four patients were male (80.0%). Each patient had a different explanation for being unrespectable, and three patients (or 60.0%) were unable to have their metastatic LNs removed. Another reason for unrespectability was the patient’s poor general condition and medical history. Neoadjuvant chemotherapy regimens were different individually while four (80.0%) patients had experienced XELOX. Median duration of chemotherapy was 6 months, ranging 1 to 10 months. After resection, pathological reports confirmed that two (40.0%) patients had metastatic carcinoma in regional LNs and 2 (40.0%) patients had adjuvant therapy. Only one patient was reported as expired and median follow-up duration was 58 months after surgery, raging 8 to 92 months.
Table 1
Surgical experiences of duodenal cancer treated with neoadjuvant chemotherapy
|
Total patients (n = 5) |
|
Age (yr) |
65 (51–81) |
|
Sex |
|
|
Male |
4 (80.0) |
|
Female |
1 (20.0) |
|
Reason for unresectability |
|
|
Pancreas invasion |
1 (20.0) |
|
Vascular invasion |
1 (20.0) |
|
Abutting vascular structure |
1 (20.0) |
|
Distant metastasis |
1 (20.0) |
|
Metastatic LN |
3 (60.0) |
|
Else |
1 (20.0) |
|
Neoadjuvant CTx regimen |
|
|
FOLFIRI |
2 (40.0) |
|
XELOX |
4 (80.0) |
|
FOLFOX |
1 (20.0) |
|
Neoadjuvant CTx duration (mon) |
6 (1–10) |
|
Operation |
|
|
PPPD |
3 (60.0) |
|
Whipple’s operation |
1 (20.0) |
|
Segmental resection |
1 (20.0) |
|
Pathological finding |
|
|
No metastasis |
3 (60.0) |
|
Metastatic carcinoma in regional LNs |
2 (40.0) |
|
Adjuvant therapy |
|
|
None |
3 (60.0) |
|
CTx and/or RTx |
2 (40.0) |
|
Survival outcome |
|
|
Alive |
2 (40.0) |
|
Death |
1 (20.0) |
|
F/U loss |
2 (40.0) |
|
Postoperative F/U duration (mon) |
58 (8–92) |

Among these patients, there were several unique cases. A patient with liver metastasis survived 92 months (follow-up lost) after Whipple’s operation along with preoperative XELOX (eight times, six months) and no postoperative therapy. A patient with open and closure history due to SMA invasion survived 44 months (follow-up lost) after segmental resection along with preoperative FOLFOX (six times, four months) and XELOX (seven times, three months), and postoperative XELOX (six times, six months) and FOLFIRI (24 times, 16 months).
Go to :

DISCUSSION
Surgery is the only know curative treatment option for duodenal cancer [
7,
8]. Usually, pancreatoduodenectomy and segmental resection are the main two options for surgery, and both methods are acceptable unless margin-free resection and adequate lymphadenectomy was obtained [
9]. Unfortunately, symptoms of duodenal cancer are nonspecific so patients usually present with advanced status, which is unresectable due to large tumor size, multiple regional metastatic LNs, tumor invading major vascular structures, and distant metastasis [
10].
In this case, there were several reasons why neoadjuvant chemotherapy was conducted before elective surgery. First, total resection of duodenal cancer was nearly impossible due to invasion to neighboring vascular structures. According to initial imaging studies, the duodenal cancer was abutting RHA (raising from SMA, which is an anatomic variation) and main PV, expecting hard resectability which is similar to the resectability concept of pancreatic ductal adenocarcinoma [
11]. Second, since several regional LN invasions were suspected, instead of resecting all suspicious LNs, reducing metastatic LNs was thought to be more advantageous for R0 resection. Third, pancreatic head invasion of the duodenal cancer was present in this patient. Even though there was some pancreatic head invasion prior to PPPD, it was anticipated that reducing the size and extent to the pancreas would be necessary for a better surgical outcome.
To the best of our knowledge, this is the first report of several neoadjuvant chemotherapy reports for duodenal cancer in South Korea, particularly from an institution with advanced surgical training. Cases such as DA with liver metastasis or open and closure history due to SMA invasion had favorable results, surviving longer than expected.
Several other reports also demonstrated successful cases of neoadjuvant therapies. A 60-year-old female patient with locally advanced DA had oxaliplatin and capecitabine for six cycles before pancreatoduodenectomy and confirmed as free from duodenal cancer after 34 months [
12]. A 53-year-old male patient with duodenal bulb adenocarcinoma had preoperative S-1 with oxaliplatin neoadjuvant chemotherapy and achieved complete pathological response [
13]. Onkendi et al. [
5] reported 10 patients with unresectable primary or recurrent DA who undergone neoadjuvant therapy. Among these patients, 40% patients had neoadjuvant chemotherapy while 60% patients had additional radiotherapy. The median overall survival was 19 months, and five patients were reported alive at 18 to 83 months after resection. Furthermore, 60% of these 5 patients effectively underwent neoadjuvant therapy for downstaged locally unresectable condition. An analysis of 2,956 DA patients in the National Cancer Database found that those who received systemic therapy had longer median overall survival than those who only underwent surgery, and those who received neoadjuvant therapy had survival that was comparable to that of those who received adjuvant therapy [
14].
According to our experience, there might be a specific regimen which is favorable for neoadjuvant therapy. Regimen alternation might result better response and improve curable resection rates. Because duodenal cancer is rare and neoadjuvant therapy is not commonly practiced, well-designed studies are lacking. Due to small patient size, various regimens and diverse clinical characteristics of patients are not considered individually and are analyzed as mixed up. Thus, more specifically designed studies should be conducted to standardize appropriate patient selection and regimen choice for neoadjuvant chemotherapy in duodenal cancer.
In spite of limited clinical practice of duodenal cancer treated by neoadjuvant treatment, recent studies highlighted the prognostic improvement of neoadjuvant chemotherapy followed by radical resection in borderline resectable pancreatic cancer and locally advance pancreatic cancer [
15-
17]. Although it is difficult to propose a precise indication for preoperative chemotherapy in DA, we examined a variety of factors that were common to these patients. Except one expired patient, four patients had received XELOX before surgery. Among these four patients, one patient who was initially inoperable due to SMA invasion had undergone adjuvant chemoradiotherapy and follow-up lost after 44 months, while other three patients survived nearly more than five years. According to these findings, we carefully suggested an indication for neoadjuvant chemotherapy as follows; inoperable DA due to regional LN metastases, and/or vascular structure (except SMA) invasion, and/or regional pancreas invasion. Also, better prognosis was expected from patients who had favorable response to XELOX. However, regarding this issue, further study based on multicenter collaboration is mandatory.
In conclusion, according to our experience, neoadjuvant chemotherapy is capable to change unresectable duodenal cancer to a resectable status. Additionally, it may be an alternative for patients with duodenal cancer who are unable to have procedures due to poor general health, while waiting till the patient regains operative state. At last, it suggested that aggressive and longer preoperative chemotherapy, maybe more than six months, should be done to obtain successful results. Additionally, it will be crucial to regularly monitor the growth of the cancer and the effectiveness of the treatment, then choose a different regimen if necessary.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download