Abstract
Littoral cell angiomas are rare vascular tumors of the spleen. Because of their rarity, unclear etiopathogenesis, and association with other malignancies, these tumors can pose diagnostic and therapeutic challenges. Due to paucity of published literature on this entity often limited to case reports, relevant data on this topic were procured and synthesized with the aid of a comprehensive Medline search in addition to oncologic, pathologic, radiologic, and surgical literature review on littoral cell angiomas. This article provides an in-depth review into postulated etiopathogenesis, pathology, clinical manifestations, associated malignancies, and prognostic features of littoral cell angiomas.
Littoral cell angioma (LCA) is a primary vascular neoplasm of the spleen with less than 200 cases reported in literature to date. LCA is a rare benign lesion, taking its origin from littoral cells (Latin: 'littoralis' meaning seashore) of the trabeculated mesh in the reticuloendothelial system lining splenic red pulp sinuses. First described in 1991 by Falk et al. [1], LCA tumor cells display a unique myriad of endothelial-histiocytic immunological phenotype, the key to a definitive diagnosis of this tumor. LCA has been reported in every age group, from a 26-day old neonate to a woman aged 83 years, without any sex-based predilection [2,3]. Two variants of LCA (littoral cell hemangioendothelioma and littoral cell angiosarcoma) have malignant potential and hence warrant more understanding into the etiopathogenesis, management, and follow-up screening of this seemingly benign lesion.
Most patients are asymptomatic, with LCA presenting as an incidental radiological finding.
While some present with vague clinical complaints such as abdominal pain, fever, fatigue, bleeding diathesis, or weight loss, further workup of patients usually reveal splenomegaly, anemia, and thrombocytopenia (Fig. 1). This can be probably explained by hemophagocytosis (removal, destruction, and subsequent siderosis) by this neoplasm. It is also supported by the fact that these resolve after splenectomy [4]. Magnetic resonance imaging (MRI) characteristics vary with the amount of hemosiderin within the tumor. Unenhanced and contrast-enhanced sequences of MRI can aid distinguish LCA from other angiosarcomatous (potentially malignant) vascular lesions [5]. However, definitive diagnosis often warrants tissue diagnosis. A high biopsy-related complication rate (10.3%) reported for patients with refractory thrombocytopenia or vascular splenic neoplasms and fear of seeding malignant cells at the time of percutaneous splenic biopsy have led to splenectomy being both diagnostic and therapeutic [6]. Splenectomy can be performed both laparoscopically or in open fashion with attention to avoid splenic capsular disruption and tumor spillage.
Grossly, LCA is a meshwork of solid tissue, dilated cavernous vessels, and numerous narrow vascular channels with occasional microthrombi in lumen in a very organoid form reminiscent of normal sinuses in the spleen, appearing as well-defined blood or hemosiderin-filled spongy lesions in the spleen, existing either as solitary or multiple lesions, ranging in size from 1 mm to 21 cm (Fig. 2) [7,8]. These are well demarcated without capsule, compressing against the normal splenic parenchyma (Fig. 3). LCA is characterized by positive reactivity to a myriad of endothelial and histiocytic cell markers, including D56, synaptophysin, CD10, alpha-1-antitrypsin, CD31, CD163, CD4, CD8, CD68, BMA120, patchy CD34, ERG, CD68, CD21, vWF/FVIII related antigen, D2-40, Mac-387, Ham-56, vimentin and lysozyme, LYVE-1, FLI-1, vascular endothelial growth factor receptor (VEGFR-2), VEGFR-3, claudin-5, and LMO2. While WT1 is positive in normal endothelium (and normal littoral cells), LCA is WT-1 negative, emphasizing immunophenotypic transformation of neoplastic cells from normal cells [9].
Although the etiopathogenesis of LCA is unclear, its association with a myriad of conditions (apart from malignancies) including congenital and immunologic conditions points towards the role of a dysregulated immune response (Table 1) [2,8,10-22]. Immunosuppression was a notable risk factor in several of reported LCA cases that included renal transplantation [10,11], steroid refractory immune thrombocytopenic purpura [12], Myelodysplastic syndrome on pulse steroid therapy [13], systemic lupus erythematosus [14], pulmonary sarcoidosis [15], and Ehlers-Danlos syndrome on biologic immunosuppression for psoriatic arthritis [16], although the duration of immunosuppression and the kind of drugs varied widely amongst them. It has been hypothesized that LCA and the resulting splenomegaly can create an altered immune state of the body, with resolution of leukocytosis after excision being proof of same [16]. Varying levels of tumor necrosis factor (TNF)-alpha have also been speculated to be the cause of LCA [15]. Several reported cases in the literature had co-existing LCA with inflammatory bowel disease, further strengthening the link of LCA with TNF-alpha [23-25].
LCA is frequently associated with a wide range of malignancies, including epithelial, mesenchymal, and hematological tumors. To date, there have been at least 24 reports of LCA associated with a malignancy (8 patients had a pre-existing malignancy years before; 13 at the time of diagnosis of LCA) (Table 2) [3,5,7,8,13,17,23,26-40]. The wide range of tumors associated with LCA makes it difficult to draw any conclusions regarding its possible pathogenesis. Genetic associations (for instance, with BRCA1 or BRCA2—being frequently associated with breast, prostate, and pancreatic cancer) and familial clusters (even though a case of siblings was reported—one with LCA, and another with primary splenic angiosarcoma) have not been established for LCA [41]. The follow-up in reported cases of LCA in the literature varied from two weeks to ten years, with a mean follow-up of 15 months.
In spite of several associations with malignancies at the time or before the diagnosis of LCA, only three reported cases in the literature (all above the age of 61 years) have described metastatic or recurrent LCA post splenectomy [42-44]. Two [42,43] of these studies reported atypical histology (presence of solid areas with/without necrotic foci) in the primary splenic LCA with subsequent metastatic histology labelling it as littoral cell hemangioendothelioma. Thus, the histologic diagnosis of LCA in primary splenic lesion is questionable in these two cases. Metastases in these cases appeared at eight and four years after the index case, involving the liver, periaortic, and retroperitoneal lymph nodes. Patients survived for less than four months. The third patient [44] was the only reported case of true LCA with recurrence noted 10 years later in the liver and periportal lymph nodes. The patient underwent chemotherapy for 21 months before death.
LCA by the sheer size of the spleen or history of associated malignancies/immunological conditions at the time of LCA diagnosis poses a diagnostic dilemma which often warrants a splenectomy [45]. Precise histologic diagnosis of LCA carries a good prognosis. Presence of ominous histological features such as abnormal architecture, solid areas, necrotic foci, or nuclear atypia should raise the suspicion for alternative diagnosis including littoral cell hemangioendothelioma and littoral cell angiosarcoma and warrant a closer follow-up protocol for recurrence/metastasis. Owing to the rarity of splenic angiomas and wide spectrum of associated malignancies, implementation of a generalized screening protocol is generally impractical. Continued efforts are needed to better understand the role of immune dysregulation and facilitate early detection of associated malignancies. The association of these splenic lesions with other visceral malignancies calls for close clinical follow-up and surveillance which are not very well defined yet [46,47].
REFERENCES
1. Falk S, Stutte HJ, Frizzera G. 1991; Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 15:1023–1033. DOI: 10.1097/00000478-199111000-00001. PMID: 1928554.
2. Gakenheimer-Smith L, Mohlman J, VandenHeuvel K, Jackson WD, Thomsen W, Stevenson A, et al. 2018; A novel presentation of littoral cell angioma and lymphatic malformations in a neonate. Pediatrics. 141(Suppl 5):S520–S525. DOI: 10.1542/peds.2017-2782. PMID: 29610184.

3. Priego P, Rodríguez Velasco G, Griffith PS, Fresneda V. 2008; Littoral cell angioma of the spleen. Clin Transl Oncol. 10:61–63. DOI: 10.1007/s12094-008-0155-3. PMID: 18208795.

4. Español I, Lerma E, Fumanal V, Palmer J, Roca M, Domingo-Albós A, et al. 2000; Littoral cell angioma with severe thrombocytopenia. Ann Hematol. 79:46–49. DOI: 10.1007/s002770050009. PMID: 10663622.

5. Schneider G, Uder M, Altmeyer K, Bonkhoff H, Gruber M, Kramann B. 2000; Littoral cell angioma of the spleen: CT and MR imaging appearance. Eur Radiol. 10:1395–1400. DOI: 10.1007/s003300000345. PMID: 10997426.

6. Zhang YH, Liu LM, Wang WP, Ding H, Wang XN, Xia HS. 2013; Littoral cell angioma of the spleen: sonographic-pathologic comparison. J Ultrasound Med. 32:691–697. DOI: 10.7863/jum.2013.32.4.691. PMID: 23525396.
7. Veillon DM, Williams RB, Sardenga LJ, Harrison GK, Cotelingam JD. 2000; 'Little' littoral cell angioma of the spleen. Am J Surg Pathol. 24:306–307. DOI: 10.1097/00000478-200002000-00023. PMID: 10680900.

8. Truong V, Finch R, Martin B, Buzacott K, Singh M, Patel B. 2019; Littoral cell angioma of spleen. ANZ J Surg. 89:E158–E159. DOI: 10.1111/ans.14193. PMID: 29024432.

9. O'Malley DP, Kim YS, Weiss LM. 2015; Distinctive immunohistochemical staining in littoral cell angioma using ERG and WT-1. Ann Diagn Pathol. 19:143–145. DOI: 10.1016/j.anndiagpath.2015.02.007. PMID: 25792460.
10. Tan YM, Chuah KL, Wong WK. 2004; Littoral cell angioma of the spleen. Ann Acad Med Singap. 33:524–526.
11. Mühlfeld AS, Eitner F, Perez-Bouza A, Knuechel R, Heintz B, Floege J. 2008; Littoral cell angioma of the spleen mimicking posttransplantation lymphoma in a 63-year-old renal transplant patient. Am J Kidney Dis. 52:e11–e14. DOI: 10.1053/j.ajkd.2008.01.033. PMID: 18479795.

12. Gardner JA, Devitt K. 2017; Incidental littoral cell angioma in refractory immune thrombocytopenic purpura. Blood. 129:1564. DOI: 10.1182/blood-2016-10-748772. PMID: 28302694.

13. Erçin C, Gürbüz Y, Hacihanefioğlu A, Turgut Karakaya A. 2005; Multiple littoral cell angioma of the spleen in a case of myelodysplastic syndrome. Hematology. 10:141–144. DOI: 10.1080/10245330400026121. PMID: 16019460.

14. Mac New HG, Fowler CL. 2008; Partial splenectomy for littoral cell angioma. J Pediatr Surg. 43:2288–2290. DOI: 10.1016/j.jpedsurg.2008.07.031. PMID: 19040956.

15. Cordesmeyer S, Pützler M, Titze U, Paulus H, Hoffmann MW. 2011; Littoral cell angioma of the spleen in a patient with previous pulmonary sarcoidosis: a TNF-α related pathogenesis? World J Surg Oncol. 9:106. DOI: 10.1186/1477-7819-9-106. PMID: 21929754. PMCID: PMC3187736.

16. Gorman D, Bergstrom R. 2016; Littoral cell angioma in a patient with Ehlers-Danlos syndrome on biologic immunosuppression for psoriatic arthritis. Int J Case Rep Images. 7:800–804.
17. Collins GL, Morgan MB, Taylor FM 3rd. 2003; Littoral cell angiomatosis with poorly differentiated adenocarcinoma of the lung. Ann Diagn Pathol. 7:54–59. DOI: 10.1053/adpa.2003.50009. PMID: 12616475.

18. Roldan-Vasquez E, Roldan-Vasquez A, Jarrin-Estupiñan X, Roldan-Crespo J. 2021; Case report: Infrequent littoral cell angioma of the spleen. Int J Surg Case Rep. 85:106242. DOI: 10.1016/j.ijscr.2021.106242. PMID: 34333257. PMCID: PMC8346658.

19. Ziske C, Meybehm M, Sauerbruch T, Schmidt-Wolf IG. 2001; Littoral cell angioma as a rare cause of splenomegaly. Ann Hematol. 80:45–48. DOI: 10.1007/s002770000223. PMID: 11233776.

20. Gupta MK, Levin M, Aguilera NS, Pastores GM. 2001; Littoral cell angioma of the spleen in a patient with Gaucher disease. Am J Hematol. 68:61–62. DOI: 10.1002/ajh.1151. PMID: 11559940.

21. Forest F, Duband S, Clemenson A, Peoc'h M. 2010; Traumatic subcapsular splenic hematoma revealing littoral cell angioma and Gaucher's disease. Ann Hematol. 89:1061–1062. DOI: 10.1007/s00277-010-0909-1. PMID: 20155266.

22. Antón-Pacheco J, Ayuso RM, Cano I, Martinez MA, Cuadros J, Berchi FJ. 2000; Splenic littoral cell angioma in an infant. J Pediatr Surg. 35:508–509. DOI: 10.1016/S0022-3468(00)90225-2. PMID: 10726700.

23. Sarandria JJ, Escano M, Kamangar F, Farooqui SO, Montgomery E, Cunningham SC. 2014; Littoral cell angioma: gastrointestinal associations. Gastrointest Cancer Res. 7:63–64.
24. Johansson J, Björnsson B, Ignatova S, Sandström P, Ekstedt M. 2015; Littoral cell angioma in a patient with Crohn's disease. Case Rep Gastrointest Med. 2015:474969. DOI: 10.1155/2015/474969. PMID: 25705528. PMCID: PMC4326338.

25. Nagarajan P, Cai G, Padda MS, Selbst M, Kowalski D, Proctor DD, et al. 2011; Littoral cell angioma of the spleen diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy. Diagn Cytopathol. 39:318–322. DOI: 10.1002/dc.21384. PMID: 21488173.

26. Bhatt S, Huang J, Dogra V. 2007; Littoral cell angioma of the spleen. AJR Am J Roentgenol. 188:1365–1366. DOI: 10.2214/AJR.06.1157. PMID: 17449783.

27. Pilz JB, Sperschneider T, Lutz T, Loosli B, Maurer CA. 2011; Littoral cell angioma in main and accessory intrapancreatic spleen presenting as splenic rupture. Am J Surg. 201:e15–e17. DOI: 10.1016/j.amjsurg.2009.11.013. PMID: 20409532.

28. Steensma DP, Morice WG. 2000; Littoral cell angioma associated with portal hypertension and resected colon cancer. Acta Haematol. 104:131–134. DOI: 10.1159/000039747. PMID: 11154990.

29. Opatrny V, Treska V, Waloschek T, Molacek J. 2020; Littoral cell angioma of the spleen: a case report. SAGE Open Med Case Rep. 8:2050313X20959874. DOI: 10.1177/2050313X20959874. PMID: 33088569. PMCID: PMC7543100.

30. Bhavsar T, Wang C, Huang Y, Karachristos A, Inniss S. 2011; Littoral cell angiomas of the spleen associated with solid pseudopapillary tumor of the pancreas. World J Gastrointest Pathophysiol. 2:53–56. DOI: 10.4291/wjgp.v2.i3.53. PMID: 21860837. PMCID: PMC3158892.

31. Heese J, Bocklage T. 2000; Specimen fine-needle aspiration cytology of littoral cell angioma with histologic and immunohistochemical confirmation. Diagn Cytopathol. 22:39–44. DOI: 10.1002/(SICI)1097-0339(200001)22:1<39::AID-DC11>3.0.CO;2-Q.

32. Floyd JD, Kaplan PA, Sauter ER, Perry MC, Doll DC. 2005; Patients with unusual bladder malignancies and a rare cause of splenomegaly. Case 3. Littoral cell angioma of the spleen in a patient with previous lymphoma. J Clin Oncol. 23:4460–4462. DOI: 10.1200/JCO.2005.05.066. PMID: 15994157.

33. Melzer N, Barth PJ, Müller KM, Foss HD, Krug U, Schilling M, et al. 2012; Rapidly progressive B-cell dominated inflammatory neuropathy and littoral cell angioma of the spleen associated with plasmablastic B-cell lymphoma. Leuk Lymphoma. 53:1242–1244. DOI: 10.3109/10428194.2011.640677. PMID: 22098375.

34. Marzetti A, Messina F, Prando D, Verza LA, Vacca U, Azabdaftari A, et al. 2015; Laparoscopic splenectomy for a littoral cell angioma of the spleen: case report. World J Clin Cases. 3:951–955. DOI: 10.12998/wjcc.v3.i11.951. PMID: 26601099. PMCID: PMC4644898.

35. Chatelain D, Bonte H, Guillevin L, Balladur P, Flejou JF. 2002; Small solitary littoral cell angioma associated with splenic marginal zone lymphoma and villous lymphocyte leukaemia in a patient with hepatitis C infection. Histopathology. 41:473–475. DOI: 10.1046/j.1365-2559.2002.14313.x. PMID: 12405918.

36. Yano H, Imasato M, Monden T, Okamoto S. 2003; Hand-assisted laparoscopic splenectomy for splenic vascular tumors: report of two cases. Surg Laparosc Endosc Percutan Tech. 13:286–289. DOI: 10.1097/00129689-200308000-00014. PMID: 12960796.
37. Harmon RL, Cerruto CA, Scheckner A. 2006; Littoral cell angioma: a case report and review. Curr Surg. 63:345–350. DOI: 10.1016/j.cursur.2006.06.011. PMID: 16971207.

38. Shah S, Wasnik A, Pandya A, Bude RO. 2011; Multimodality imaging findings in image-guided biopsy proven splenic littoral cell angioma: series of three cases. Abdom Imaging. 36:735–738. DOI: 10.1007/s00261-011-9697-x. PMID: 21318377.

39. Mohan V, Jones RC, Drake AJ 3rd, Daly PL, Shakir KM. 2005; Littoral cell angioma presenting as metastatic thyroid carcinoma to the spleen. Thyroid. 15:170–175. DOI: 10.1089/thy.2005.15.170. PMID: 15753678.

40. Yaney A, Jones D, Perry KA, Jhawar SR. 2022; Rare case of littoral cell angioma recognised on CT simulation for adjuvant radiation treatment for early stage breast cancer. BMJ Case Rep. 15:e248167. DOI: 10.1136/bcr-2021-248167. PMID: 35131803.

41. Kranzfelder M, Bauer M, Richter T, Rudelius M, Huth M, Wagner P, et al. 2012; Littoral cell angioma and angiosarcoma of the spleen: report of two cases in siblings and review of the literature. J Gastrointest Surg. 16:863–867. DOI: 10.1007/s11605-011-1773-6. PMID: 22068970.

42. Ben-Izhak O, Bejar J, Ben-Eliezer S, Vlodavsky E. 2001; Splenic littoral cell haemangioendothelioma: a new low-grade variant of malignant littoral cell tumour. Histopathology. 39:469–475. DOI: 10.1046/j.1365-2559.2001.01242.x. PMID: 11737304.

43. Fernandez S, Cook GW, Arber DA. 2006; Metastasizing splenic littoral cell hemangioendothelioma. Am J Surg Pathol. 30:1036–1040. DOI: 10.1097/00000478-200608000-00016. PMID: 16861977.

44. Takayoshi K, Doi G, Tsuruta N, Yoshihiro T, Nio K, Tsuchihashi K, et al. 2018; Successful chemotherapeutic treatment for metastatic littoral cell angioma: a case report. Medicine (Baltimore). 97:e0378. DOI: 10.1097/MD.0000000000010378. PMID: 29642193. PMCID: PMC5908586.
45. Hu ZQ, A YJ, Sun QM, Li W, Li L. 2011; The splenic littoral cell angioma in China: a case report and review. World J Surg Oncol. 9:168. DOI: 10.1186/1477-7819-9-168. PMID: 22172167. PMCID: PMC3271992.

46. Wang Z, Zhang L, Zhang B, Mu D, Cui K, Li S. 2015; Hemangioendothelioma arising from the spleen: a case report and literature review. Oncol Lett. 9:209–212. DOI: 10.3892/ol.2014.2693. PMID: 25435960. PMCID: PMC4246698.

47. Wheelwright M, Spartz EJ, Skubitz K, Yousaf H, Murugan P, Harmon JV. 2021; Primary angiosarcoma of the spleen, a rare indication for splenectomy: a case report. Int J Surg Case Rep. 82:105929. DOI: 10.1016/j.ijscr.2021.105929. PMID: 33957408. PMCID: PMC8113851.

Fig. 1
Early contrast-enhanced helical computed tomography scan showing enlarged spleen containing innumerable small, focal, low-density lesions.
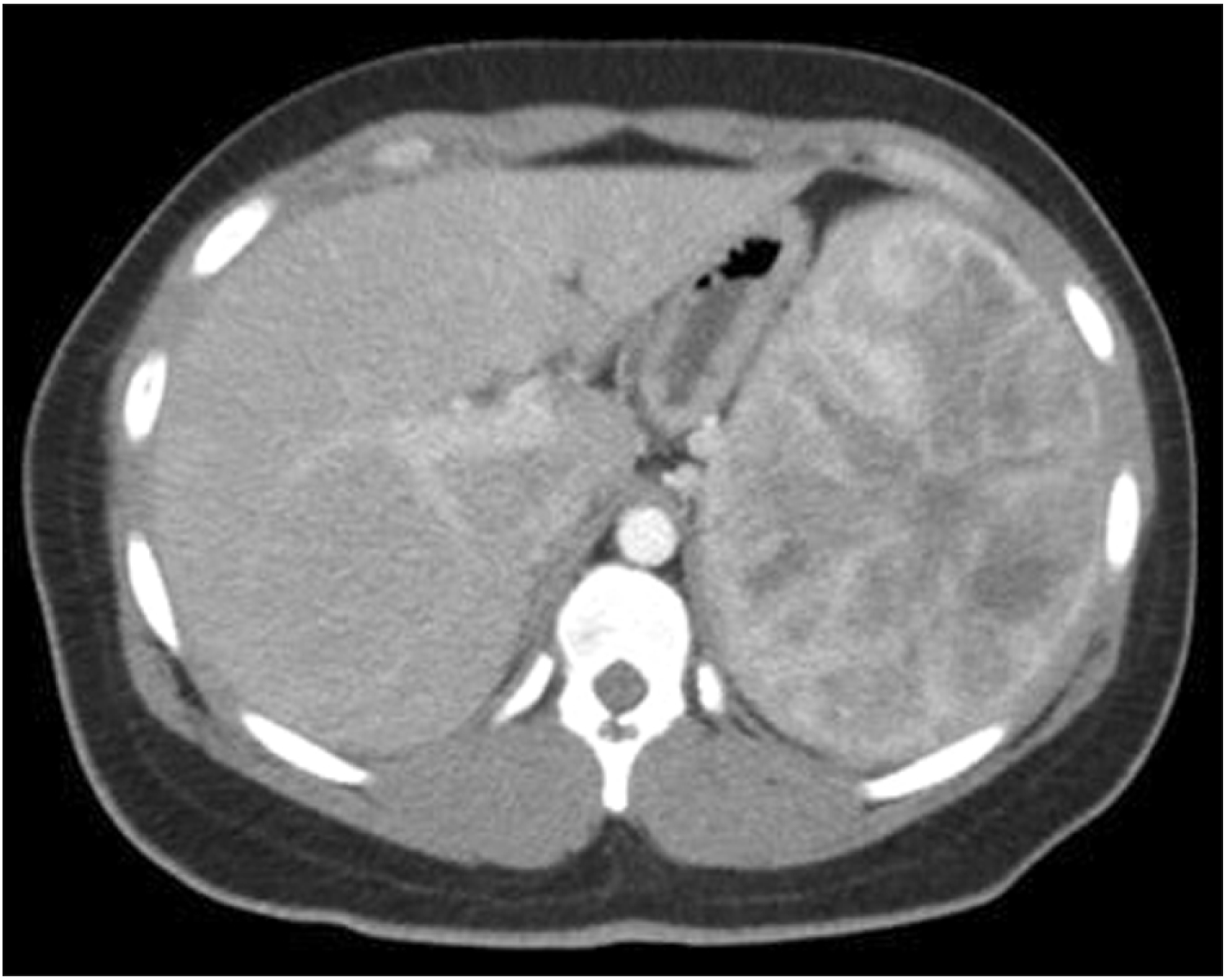
Fig. 2
Serial sections of spleen showing that the parenchyma is notable for multiple hemorrhagic cystic lesions diffusely spread throughout the parenchyma.
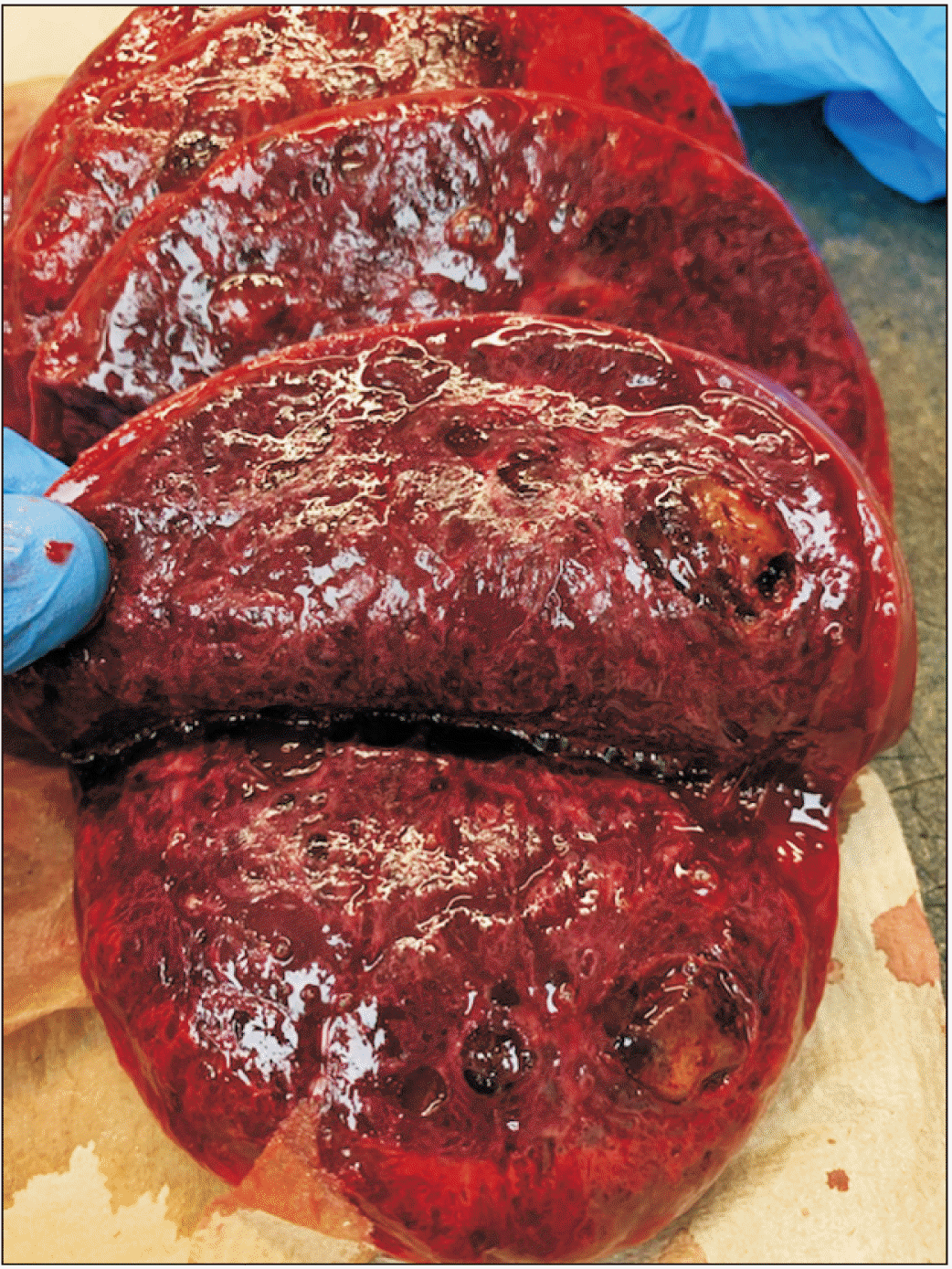
Fig. 3
Histologic specimen showing normal splenic parenchyma (large arrow) and adjacent vascular proliferation of cavernous, blood-filled spaces (small arrow) (H&E, ×20).
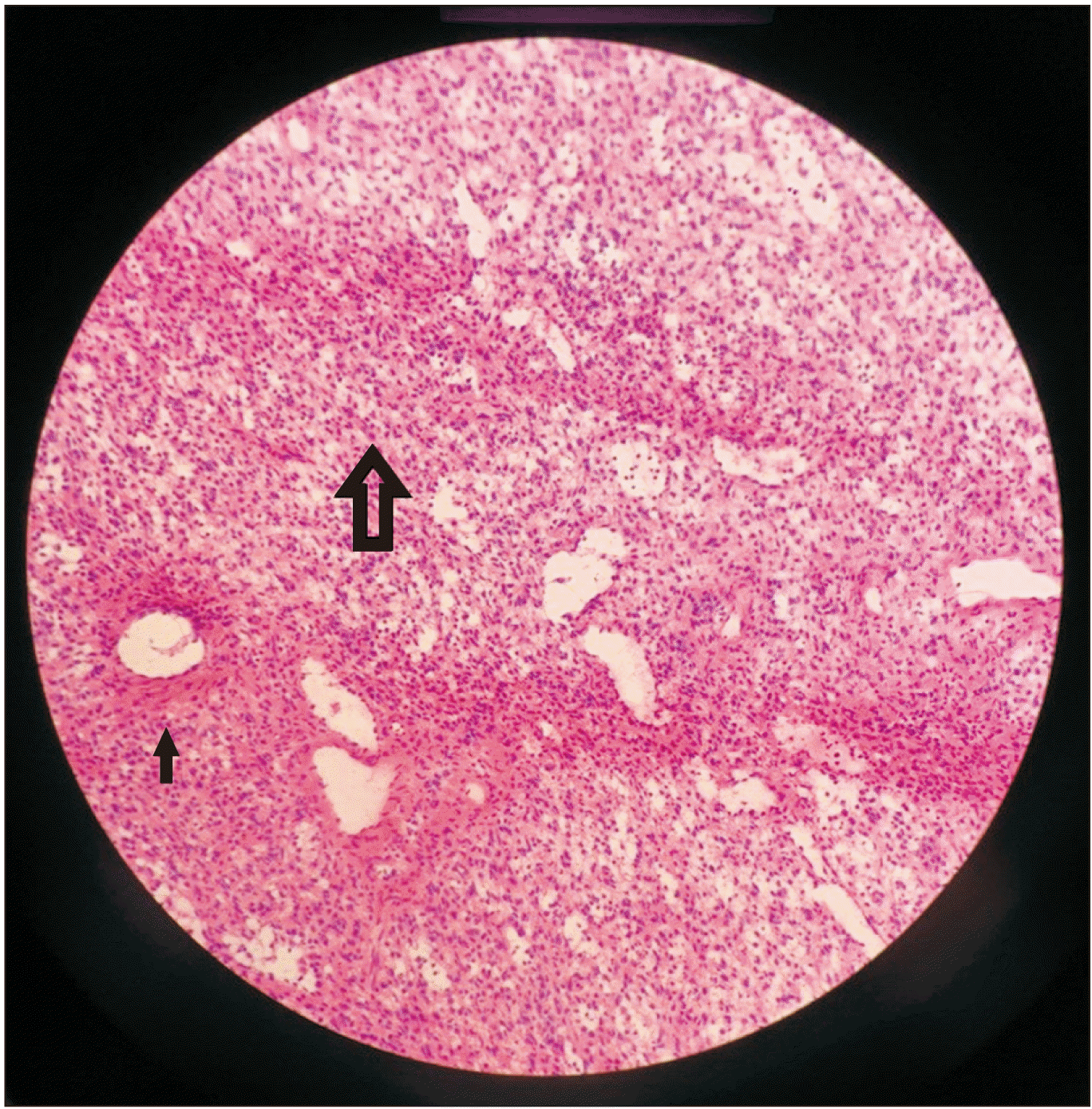
Table 1
Speculated etiopathogenesis of littoral cell angioma (LCA)
| Speculated pathogenic factor | Reference | Condition associated with LCA |
|---|---|---|
| Immune dysregulation | Tan et al. (2004) [10] | Immunosuppression (renal transplant) |
| Erçin et al. (2005) [13] | Immunosuppression (Myelodysplastic syndrome) | |
| Mühlfeld et al. (2008) [11] | Immunosuppression (renal transplant) | |
| Mac New and Fowler (2008) [14] | Immunosuppression (SLE) | |
| Cordesmeyer et al. (2011) [15] | Immunosuppression (Pulmonary sarcoidosis) | |
| Gorman and Bergstrom (2016) [16] | Immunosuppression; Connective Tissue Disorder (Ehler Danlos) | |
| Gardner and Devitt (2017) [12] | Immunosuppression (steroid refractory ITP) | |
| Roldan-Vasquez et al. (2021) [18] | Hypothyroidism and diabetes mellitus | |
| Environmental Carcinogen | Ziske et al. (2001) [19] | Worked in a chemical industry |
| Collins et al. (2003) [17] | Exposure to boiler fumes of ship during WW2 | |
| Macrophage Colony Stimulating Factor | Gupta et al. (2001) [20] | Gaucher’s disease |
| Forest et al. (2010) [21] | Gaucher’s disease | |
| Congenital | Gakenheimer-Smith et al. (2018) [2] | LCA in an infant |
| Antón-Pacheco et al. (2000) [22] | LCA in an infant | |
| Reactive disorder | Truong et al. (2019) [8] | Clear cell RCC |
Table 2
Associated malignancies with littoral cell angioma (LCA)
| Malignancy | Year | Author | Age/sex | Time of diagnosis of associated malignancy |
|---|---|---|---|---|
| Malignant melanoma | 2000 | Schneider et al. [5] | 66/M | At the same time |
| 2007 | Bhatt et al. [26] | 56/M | (Not mentioned) | |
| 2011 | Pilz et al. [27] | 73/M | 25 years before | |
| Colon adenocarcinoma | 2000 | Steensma and Morice [28] | 54/F | 3 years before |
| 2020 | Opatrny et al. [29] | 53/F | At the same time | |
| Pancreatic tumors | ||||
| Malignant cystic neuroendocrine neoplasm of pancreas | 2000 | Veillon et al. [7] | 53/M | At the same time |
| Pseudopapillary tumor of the pancreas | 2011 | Bhavsar et al. [30] | 30/F | At the same time |
| Lymphoma | ||||
| CNS large cell lymphoma | 2000 | Heese and Bocklage [31] | 33/M | At the same time |
| Nodular Sclerosis Hodgkin’s & diffuse large T cell lymphoma | 2005 | Floyd et al. [32] | 42/M | (Not mentioned) |
| Myelodysplastic syndrome | 2005 | Erçin et al. [13] | 36/F | 1 year after |
| Plasmablastic B cell lymphoma | 2012 | Melzer et al. [33] | 67/M | At the same time |
| Chronic myeloproliferative disorder | 2015 | Marzetti et al. [34] | 79/F | At the same time |
| Splenic marginal zone lymphoma and villous lymphocytic leukaemia | 2002 | Chatelain et al. [35] | 50/F | At the same time |
| Urologic tumors | ||||
| Seminoma of testis | 2003 | Yano et al. [36] | 33/M | 1 year before |
| Transitional cell carcinoma bladder | 2006 | Harmon et al. [37] | 74/F | 2 years before |
| Renal cell carcinoma | 2011 | Shah et al. [38] | 78/M | 2 years before |
| Non-metastatic Prostate cancer | 2012 | Melzer et al. [33] | 67/M | 2 years before |
| Clear cell RCC | 2019 | Truong et al. [8] | 42/M | At the same time |
| Lung adenocarcinoma | 2003 | Collins et al. [17] | 64/M | 1 year after |
| Papillary thyroid carcinoma | 2005 | Mohan et al. [39] | 25/M | 2 years before |
| Sarcoma | 2014 | Sarandria et al. [23] | 32/M | At the same time |
| Thigh leiomyosarcoma | 2007 | Bhatt et al. [26] | 56/M | At the same time |
| Scapular sarcoma | 2008 | Priego et al. [3] | 55/F | 13 years before |
| Giant cell tumor of femur | 2011 | Shah et al. [38] | 57/M | At the same time |
| Breast cancer | 2022 | Yaney et al. [40] | 63/F | At the same time |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download