Abstract
An accessory submandibular gland is a rare variation. As such, there is limited literature regarding the embryology, anatomy, variations, clinical imaging, and pathology of the accessory submandibular gland. In this article, we review the existing literature on the accessory submandibular gland from clinical and anatomical perspectives. The goal of this review is to provide comprehensive knowledge of this variation which can be useful for oral and maxillofacial/head and neck surgeons, radiologists, and anatomists. Within this review, the embryologic origin as well as the anatomy of the accessory submandibular gland is detailed. Several imaging modalities which can be used to visualize the accessory submandibular gland are outlined as well as its variations. Lastly, this review investigates several reported clinical considerations regarding the accessory submandibular gland including sialoliths, Wharton’s duct obstruction, and pleomorphic adenoma.
The salivary glands associated with salivation can be categorized as major and minor glands. The major salivary glands include the parotid, submandibular, and sublingual glands. The minor salivary glands are located throughout the oral mucosa e.g., superior and inferior labial, buccal, palatine, retromolar (molar), and lingual glands [1-5]. The major salivary glands contribute to about 90% of total saliva secretions, while the minor salivary glands contribute the remaining 10% [6]. The submandibular gland is the second largest of the major salivary glands and anatomically includes both superficial and deep lobes which are separated by the mylohyoid muscle [7, 8]. It is a paired, encapsulated gland that is typically located within the submandibular triangle [7]. The capsule which surrounds the submandibular gland is a continuation of the investing layer of the deep cervical fascia [7]. The main excretory duct of the submandibular gland is referred to as Wharton’s duct and this measures approximately 5 cm in length and 1.5 mm in diameter [7]. This duct travels to the anterior part of the floor of the oral cavity, where it releases saliva at the sublingual caruncle, located on both sides of the lingual frenulum [7]. There are several congenital anomalies related to the submandibular glands (SMG) including an accessory submandibular gland [9]. Accessory salivary tissue, and more specifically, an accessory salivary gland, is defined as an additional component or detached part of the major salivary or submandibular salivary gland [10]. In this article, we present a literature review of the accessory submandibular gland from a clinical and anatomical perspective.
A database search using PubMed and Google Scholar was conducted without any language limitations. The following keywords were used in the search: “accessory submandibular gland,” “duplicated submandibular gland,” and “ectopic submandibular gland.” After the initial search, full-text assessment was performed.
The SMG are endodermal in origin and typically originate late in the fifth to sixth week of development [11, 12]. At this time, the epithelium of the medial paralingual groove, which is located at the floor of the mouth, begins to thicken [13]. This area corresponds to the primordium of the SMG parenchyma [13]. At the end of the sixth week, proliferation and invagination of the epithelial condensation occurs [13]. This condensed mesenchyme begins to assist with the formation of lobes [13]. At week seven within the “canalicular” stage, the lumen of the SMG duct begins to appear [13]. The main duct of the SMG is often referred to as Wharton’s duct [9]. During the eighth week, nerve branches from neighboring submandibular ganglion begin to innervate the glandular parenchyma [13].
Congenital anomalies related to the SMG are rare, however, these can include cases such as imperforate duct and duplications [9]. An accessory submandibular gland is an additional anomaly which may occur. Typically, this occurs roughly around the seventh week of development alongside the development of Wharton’s duct [9]. As the duct develops, it may invaginate in two separate locations or premature ventral branching may occur [9]. In these instances, an accessory submandibular gland along with an accessory duct may form [9].
Typically, salivary tissue in the oral cavity, pharynx, or upper airway that develops separately from the typical major and minor salivary glands is classified as “heterotopic” tissue [14-16]. This definition contrasts from that of accessory salivary tissue [14]. Accessory salivary tissue, and more specifically, an accessory salivary gland, is defined as an additional component or detachment from a major salivary gland [10]. This detachment occurs along the external duct of main salivary gland [10].
The accessory submandibular gland is considered to be extremely rare (Fig. 1) [15]. From a histological perspective, the tissue of the accessory gland bears little to no differences from the main or “normal” salivary glands (Fig. 2) [10]. In one case study, Bryan et al. [15] described the accessory submandibular gland as comparable to the normal submandibular gland, with the exception of mildly dilated ducts. The submandibular gland is composed of both serous and mucous cells, however, serous cells are the dominant cell-type [17]. Additionally, the submandibular gland is classified as a compound acinous gland with several lobes, separated from one another with mesenchymal connective tissue and encapsulated [11].
Anatomically, the main submandibular gland can be found bilaterally within the submandibular fossa towards the medial side of the mandible, inferior to the mylohyoid line of the mandible and superior to the hyoid bone [18]. The SMG contain both a superficial and deep component as the structure curves around the posterior border of the mylohyoid muscle [18]. The submandibular duct (Wharton’s duct) emerges from the medial side of the deep component of the gland, traveling towards the sublingual caruncle which is located at the base of the frenulum of the tongue [18]. The lingual nerve can also be found in this region, passing below the submandibular duct [18]. In the literature reviewed for this publication, reports describe the accessory submandibular gland as having developed just anterior to the main SMG and being slightly smaller in size. In both cases reported by Jafek and Strife [11], the accessory gland was found anterior to and discrete from the main SMG. The size of the accessory SMG was described as round and measuring roughly 1.5 cm or 1×1.5 cm. In the same study, observations of the accessory duct within the accessory SMG were noted. In both cases, normal filling of the SMG was confirmed via sialograms and a secondary branch of Wharton’s duct (an accessory duct) was present and discrete from the main SMG [11].
Although there is limited literature regarding the innervation of this anomaly, for this review we assume that the innervation of the accessory submandibular gland would be the same as the main submandibular gland.
Sympathetic stimulation of the submandibular gland originates from the superior cervical ganglion [7]. Sympathetic nerve cell bodies located within this ganglion extend postganglionic fibers which travel alongside branches of the external carotid artery to reach the submandibular gland [7]. This stimulation functions to regulate salivary secretion as well as initiate inflammation [7, 17].
The chorda tympani branch of the facial nerve provides parasympathetic fibers to the SMG via the submandibular ganglion [7]. The chorda tympani branches from the facial nerve in the facial canal 5 mm above the stylomastoid foramen and then exits the middle ear through the pterygotympanic fissure. The chorda tympani then travels with the lingual nerve which will then give rise to preganglionic parasympathetic fibers which will synapse at the submandibular ganglion [7]. Postganglionic fibers then act on the submandibular gland, stimulating salivary secretion from the gland (among other aspects) [7].
Although there is limited literature regarding the blood supply of this anomaly, for this review assume that it’s arterial supply as well as drainage will remain the same as the main submandibular gland, as the accessory submandibular gland is defined typically as a detachment from the main submandibular gland.
The SMG typically receive their blood supply from the submental and sublingual arteries [7]. The submental artery is considered the largest branch of the facial artery. As the submental artery travels anteromedially on the inferior border of the mandible and superficially passes the mylohyoid muscle to reach the chin [19]. Throughout its course, it will give off branches to the SMG, platysma, and mylohyoid muscles [19]. The lingual artery passes through medial to the gland and can supply the gland [20].
The SMG (as well as sublingual glands) drain primarily into the submandibular lymph nodes, of which there are roughly three to six in number [7]. The submandibular nodes then drain into the deep cervical nodes (mainly, it drains into the jugulo-omohyoid and partially the jugulodigastric nodes) [18].
The primary purpose of the SMG is to contribute to the production of saliva. Saliva is produced in the human body during both a resting (unstimulated) and active (stimulated) states [6]. In an unstimulated state, the SMG produce roughly two-thirds of the total volume of whole saliva [6]. During a stimulated state, the parotid gland will become the main contributor to whole saliva, producing approximately 50% of the total volume of saliva [6].
Saliva plays a critical role in the human body, protecting structures within the oral cavity and the respiratory pathway from pathogens and irritants [6]. Moreover, in addition to its defensive role, saliva functions to mineralize teeth, restore soft tissue, lubricate the oral cavity, assist with digestion, signals the swallowing reflex and maintains a pH buffer [6].
Studies show that changes that occur with age have been observed in both parent and accessory glands. This includes disorders of the parent tissue that are defined by inflammation or neoplastic tissue [15]. One cross-sectional study of an epidemiologically stratified population, demonstrated that with increased age saliva production from the SMG decreases [22]. Moreover, the composition of saliva was found to change with age, with protein output or secretion rate declining [22].
Variations of the SMG, more specifically the accessory SMG, can be of interest to any healthcare professional who specializes in the oral cavity and submandibular region, including dentists, otolaryngologists, radiologists, head and neck surgeons, and oral and maxillofacial surgeons [23]. In one case study regarding the accessory submandibular gland, a variation was found in which the accessory gland and the main submandibular gland connected to one another forming a “horseshoe” shape [23]. This study reported that an accessory submandibular gland was found on the right side between the mylohyoid and hyoglossus muscles [23]. The main submandibular gland in this case study had a superficial and deep parts, with the deep part measuring around 5 cm. The accessory submandibular gland also measured 5 cm and was found below and parallel to the deep part of the main submandibular gland [23]. The accessory and main glands were connected at the lateral border of the geniohyoid muscles, forming the characteristic “horseshoe” shape for this variation [23]. It is important to note the pathway of the nerves found within this study. The lingual nerve was found to be passing between the deep and superficial parts of the main submandibular gland [23]. However, the lingual nerve as well as the hypoglossal nerve were both overlapped by the accessory submandibular gland [23]. Thus, the pathways of the aforementioned nerves are susceptible to compression by the SMG, which could result in unilateral altered sensation in the tongue along with weakness of movement [23]. In this case, the accessory submandibular gland formed with its own duct, however, this duct joined that of the main submandibular gland to form a common submandibular duct [23]. The common submandibular duct traveled deep to the conjunction point of the SMG at the lateral border of the geniohyoid muscle [23].
The salivary glands, and specifically the accessory submandibular gland, can be visualized through several imaging modalities. Imaging is a crucial and necessary component to confirm appropriate diagnosis as well as provide detail for pre-procedural surgical planning. Sialography is a relatively painless and common diagnostic technique used to visualize the salivary glands and their associated ducts [24].
According to Barrueco et al. [10], conventional sialography is often considered the “gold standard” for visualization of the parotid and SMG. This procedure utilizes cannulation of the ductal orifice and then the introduction of ionizing radiation and radiopaque iodinated contrast to outline the ductal structure of these glands [25]. Digital subtraction sialography (DSS) is utilized and is defined by instances in which an image taken pre-contrast is subtracted from an image captured following the introduction of the contrast material [25]. By doing so, the superposition of soft tissue and bony structures is removed [25]. DSS can depict ductal dilation, salivary sialoliths, and outline ductal strictures providing information such as location, length, and number [25]. However, there are some disadvantage to this technique including adverse reaction to contrast material, exposure to ionizing radiation, and complications related to ductal cannulation [25].
Magnetic resonance sialography (MR-Si) is an non-invasive alternative to conventional sialography which produces similar images of the salivary glands [25]. However, in contrast, this technique produces images without radiation, cannulation, or the injection of a contrast medium [25]. MR-Si utilizes the salivary secretion from the patient (stimulated by a sialagogue) in order to provide a natural contrast agent which will reveal the ductal pathway within the major salivary glands [10]. The intrinsic hyperintensity of static fluid on heavily T2-weighted images is a defining aspect of this technique [10]. MR-Si can assist with the diagnosis of sialoliths and strictures [25]. However, as with most techniques, there are disadvantages to this imaging modality. Dental amalgam can cause distortion of images, general MR imaging contraindications can occur, and the quality of imaging is largely dependent on the skill level of the radiographer [25].
In addition to the modalities outlines above, the salivary glands and their ducts can also be investigated through computed tomography (CT) sialography, which can provide detailed images including the surrounding soft tissue detail, as well as ultrasound, which is considered to be a relatively inexpensive and non-invasive option [25].
Disorders of the main salivary glands may also occur in an accessory gland. Specifically, there have been reported cases of several conditions, these cases are outlined in the following section. Please note that while occurrence of an accessory submandibular gland is rare, there are even fewer occurrences of pathology within the accessory submandibular gland. This anatomical variant is significant as it provides a possible alternative diagnosis for a submental mass and can reduce the need for excisional biopsy [11].
“Lithiasis” or the formation of stony concretions (calculi) commonly can be found in several systems of the human body, including the renal system. Lithiasis within the SMG is the most common type of lithiasis among the salivary glands, or “sialolithiasis” [10]. There are several factors that predispose patients to this condition, including but not limited to, high mucin content, alkaline pH, a high concentration of calcium or phosphate salts in salivary secretion, and the direction of the Wharton’s duct (which is the main duct of the submandibular gland) [10].
In one case report, researchers detected what is supposedly the first case of sialolithasis within an accessory submandibular gland [10]. A patient presented with intermittent pain and swelling in the left submandibular area, which worsened with eating [10]. Contrast enhanced CT images demonstrated both SMG were normal in size and in presentation, however, an unidentified soft tissue mass was found anterior to one of the SMG [10]. MR-Si was then utilized to further review and confirm that the unidentified mass presented with the same signal intensity as that of the typical submandibular gland [10]. With this confirmation, it was concluded that the appropriate diagnosis was that an accessory gland and ducts were present [10]. Moreover, the Wharton’s duct within the accessory submandibular gland developed with an abnormal course and a T2-weighted image confirmed a 6 mm hypointense mass consistent with sialolithiasis [10]. The calculus could be palpated close to the submandibular papilla and was removed through an intraoral approach [10].
Wharton’s duct, as mentioned previously, is the main excretory duct of the SMG, allowing saliva to enter the oral cavity. Obstruction of this duct can be the result of several conditions including autoimmune disorders, foreign bodies, compression due to tumor and, most commonly, sialoliths or salivary calculi.
In one unique case study, Köybaşioğlu et al. [26] reported the presence of an accessory submandibular gland causing obstruction of Wharton’s duct. In this instance, a patient may present with submandibular gustatory swelling which causes occasional discomfort. Due to symptoms seemingly aligning with a diagnosis of salivary calculi within the submandibular gland, it is crucial that physical examination as well as imaging is performed to ensure the correct diagnosis [26]. In the case study mentioned, physical examination revealed no calculi and conventional radiography showed no radiopacity within the submandibular region [26]. Submandibular sialography was then utilized and confirmed that the narrowing observed within Warton’s duct was due to salivary gland tissue found at the midportion of the duct [26]. To further support these findings, histopathologic analysis was performed and the tissue was confirmed to be salivary tissue and exhibit neither inflammation nor malignancy [26]. Periodic acid-Schiff staining was performed and revealed the tissue causing constriction of the duct mainly exhibited serous acinar glands consistent with that of submandibular gland tissue [26]. A unique aspect to this case, is that the duct of the accessory submandibular gland did not connect to the Wharton’s duct, as seen in ither reports of the accessory submandibular gland, but rather acted as an accessory lobe which compressed Wharton’s duct, mimicking salivary calculus [26].
A pleomorphic adenoma is defined as a benign tumor of the salivary glands [27]. It is the most common salivary gland tumor, representing 45% to 75% of all salivary gland tumors, and is often described as a “benign mixed tumor” due to its origin from both epithelial and myoepithelial elements [27]. While this condition most commonly effects the parotid gland, it can be found in the submandibular and minor salivary glands [27]. This tumor can be characterized by the lack of a true capsule and extensions throughout the normal gland parenchyma resembling finger-like pseudopodia [27]. Several diagnostic techniques are utilized to confirm the presence of this tumor including fine needle aspiration (FNA), core needle biopsy, CT, MRI, and ultrasound examinations. FNA and core needle biopsy are both tissue sampling procedures associated with low tumor seeding rates, however, core needle biopsy is considered to be more invasive as well as have a higher diagnostic accuracy [27].
In one case study reported by Bryan et al. [15], a pleomorphic adenoma was documented within an accessory submandibular gland. This is considered to be, to the author’s knowledge, the first known report of this occurrence. The patient highlighted in the study presented with a slowly enlarging swelling in the right upper neck which caused occasional discomfort at rest [15]. Upon physical examination, the mass was palpable unilaterally as a 1 cm mobile, firm mass in the submandibular triangle [15]. Ultrasound examination was utilized to further evaluate the mass and revealed a well-defined, round 12×9 mm hypoechoic mass next to the submandibular gland [15]. FNA confirmed that the mass was consistent with pleomorphic adenoma [15]. The tumor was removed and evaluation found that the tumor had lobules, myxoid stroma exhibiting cartilaginous differentiation, and varying cellularity [15]. Additionally, it was confirmed that this tumor arose within an accessory submandibular gland due to several connections between accessory ducts and ducts of the main submandibular gland.
Only scant literature exists regarding the accessory SMG. Given their size (1–1.5 cm), anatomical and radiological studies should be considered so that the incidence, morphology, and pathology of the accessory gland might be better understood. In addition, we thought anatomical variations of the submandibular gland should be noted [28, 29].
Acknowledgements
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind’s overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude [30]. The authors state that every effort was made to follow all local and international ethical guidelines and laws that pertain to the use of human cadaveric donors in anatomical research [31].
Notes
References
1. Iwanaga J, Haikata Y, Nakamura K, Kusukawa J, Watanabe K, Tubbs RS. 2021; An anatomical and histological study of mental nerve branches to the inferior labial glands. Surg Radiol Anat. 43:1801–4. DOI: 10.1007/s00276-021-02795-6. PMID: 34232370.

2. Iwanaga J, Nakamura K, Alonso F, Kirkpatrick C, Oskouian RJ, Watanabe K, Tubbs RS. 2018; Anatomical study of the so-called "retromolar gland": distinguishing normal anatomy from oral cavity pathology. Clin Anat. 31:462–5. DOI: 10.1002/ca.23047. PMID: 29349817.

3. Kamijo Y. 1969. Oral Anatomy. Anatom;Tokyo:
4. Shen D, Ono K, Do Q, Ohyama H, Nakamura K, Obata K, Ibaragi S, Watanabe K, Tubbs RS, Iwanaga J. 2021; Clinical anatomy of the inferior labial gland: a narrative review. Gland Surg. 10:2284–92. DOI: 10.21037/gs-21-143. PMID: 34422599. PMCID: PMC8340335.

5. Wang Z, Li W, Hong X, Su JZ, Hua H, Peng X, Lv L, Yu GY. 2016; Minor salivary glands function is decreased in hyposalivation-related diseases. Arch Oral Biol. 69:63–70. DOI: 10.1016/j.archoralbio.2016.05.012. PMID: 27243418.

6. Iorgulescu G. 2009; Saliva between normal and pathological. Important factors in determining systemic and oral health. J Med Life. 2:303–7. PMID: 20112475. PMCID: PMC5052503.
7. Grewal JS, Jamal Z, Ryan J. 2022. Anatomy, head and neck, submandibular gland [Internet]. StatPearls Publishing;Treasure Island, FL: Available from: http://www.ncbi.nlm.nih.gov/books/NBK542272/. cited 2022 May 11.
8. Ryu EJ, Kim DH. 2021; Anatomical insights of the mylohyoid for clinical procedures in dentistry. Clin Anat. 34:461–9. DOI: 10.1002/ca.23675. PMID: 32893917.

9. Gadodia A, Seith A, Neyaz Z, Sharma R, Thakkar A. 2007; Magnetic resonance identification of an accessory submandibular duct and gland: an unusual variant. J Laryngol Otol. 121:e18. DOI: 10.1017/S0022215107008602. PMID: 17517164.

10. Sanchez Barrueco A, Santillan Coello J, Sobrino Guijarro B, Villacampa Aubá JM, Cenjor Español C. 2016; Sialolithiasis in an accessory submandibular gland identified by magnetic resonance sialography. Ann Otol Rhinol Laryngol. 125:603–6. DOI: 10.1177/0003489416636128. PMID: 26961009.

11. Jafek BW, Strife JL. 1973; Accessory lobe of the submandibular gland. Radiology. 109:75–7. DOI: 10.1148/109.1.75. PMID: 4783131.

12. Priya. 2020; P DS, Anitha DN, Rajesh DE, Masthan DKMK. Embryology and development of salivary gland. Eur J Mol Clin Med. 7:764–70.
13. Quirós-Terrón L, Arráez-Aybar LA, Murillo-González J, De-la-Cuadra-Blanco C, Martínez-Álvarez MC, Sanz-Casado JV, Mérida-Velasco JR. 2019; Initial stages of development of the submandibular gland (human embryos at 5.5-8 weeks of development). J Anat. 234:700–8. DOI: 10.1111/joa.12955. PMID: 30740679. PMCID: PMC6481420.

14. Batsakis JG. 1986; Heterotopic and accessory salivary tissues. Ann Otol Rhinol Laryngol. 95(4 Pt 1):434–5. DOI: 10.1177/000348948609500422. PMID: 3740723.

15. Bryan S, Bodner L, Manor E, Brennan PA. 2013; Pleomorphic adenoma occurring outside the submandibular gland: a case report of an accessory submandibular gland. J Oral Maxillofac Surg. 71:1703–5. DOI: 10.1016/j.joms.2013.04.014. PMID: 23769461.

16. Singer MI, Applebaum EL, Loy KD. 1979; Heterotopic salivary tissue in the neck. Laryngoscope. 89:1772–8. DOI: 10.1288/00005537-197911000-00009. PMID: 502698.

17. Brazen B, Dyer J. 2022. Histology, salivary glands [Internet]. StatPearls Publishing;Treasure Island, FL: Available from: http://www.ncbi.nlm.nih.gov/books/NBK551688/. cited 2022 May 12.
18. Drake RL, Vogl W, Mitchell AWM, Gray H. 2020. Gray's anatomy for students. 4th ed. Elsevier;Philadelphia: p. 1102–5. DOI: 10.1288/00005537-197911000-00009.
19. Atamaz Pinar Y, Govsa F, Bilge O. 2005; The anatomical features and surgical usage of the submental artery. Surg Radiol Anat. 27:201–5. DOI: 10.1007/s00276-005-0317-8. PMID: 16003485.

20. Lettau J, Bordoni B. 2022. Anatomy, head and neck, lingual artery [Internet]. StatPearls Publishing;Treasure Island, FL: Available from: http://www.ncbi.nlm.nih.gov/books/NBK554513/. cited 2022 May 11.
21. Ono K, Yoshioka N, Hage D, Ibaragi S, Tubbs RS, Iwanaga J. 2021; Duplication of the external jugular vein: a language barrier of database search in classic anatomical studies. Surg Radiol Anat. 43:1721–8. Erratum in: Surg Radiol Anat 2021;43:1729-30. DOI: 10.1007/s00276-021-02717-6. PMID: 33620594.

22. Yeh CK, Johnson DA, Dodds MW. 1998; Impact of aging on human salivary gland function: a community-based study. Aging (Milano). 10:421–8. DOI: 10.1007/BF03339889. PMID: 9932146.

23. Nayak SB. 2018; Accessory submandibular salivary gland forming a "horseshoe" with the main submandibular salivary gland: a unique variation. J Craniofac Surg. 29:1376–7. DOI: 10.1097/SCS.0000000000004537. PMID: 29570527.
24. Hasson O. 2010; Modern sialography for screening of salivary gland obstruction. J Oral Maxillofac Surg. 68:276–80. DOI: 10.1016/j.joms.2009.09.044. PMID: 20116695.

25. Sobrino-Guijarro B, Cascarini L, Lingam RK. 2013; Advances in imaging of obstructed salivary glands can improve diagnostic outcomes. Oral Maxillofac Surg. 17:11–9. DOI: 10.1007/s10006-012-0327-8. PMID: 22562281.

26. Köybaşioğlu A, Ileri F, Gençay S, Poyraz A, Uslu S, Inal E. 2000; Submandibular accessory salivary gland causing Warthin's duct obstruction. Head Neck. 22:717–21. DOI: 10.1002/1097-0347(200010)22:7<717::AID-HED12>3.0.CO;2-1. PMID: 11002328.

27. Bokhari MR, Greene J. 2022. Pleomorphic adenoma [Internet]. StatPearls Publishing;Treasure Island, FL: Available from: http://www.ncbi.nlm.nih.gov/books/NBK430829/. cited 2022 May 12.
28. Nayak SB, Vasudeva SK, Pamidi N, Sirasanagandla SR. 2020; Anomalous course of facial artery through the submandibular gland and its redundant loop at the base of mandible. J Craniofac Surg. 31:2015–6. DOI: 10.1097/SCS.0000000000006539. PMID: 32472879.

29. Nayak SB, Kumar N. 2018; Lingual nerve entrapment in fused submandibular and sublingual salivary glands: a unique finding. J Craniofac Surg. 29:e677–9. DOI: 10.1097/SCS.0000000000004842. PMID: 30106809.

30. Iwanaga J, Singh V, Ohtsuka A, Hwang Y, Kim HJ, Moryś J, Ravi KS, Ribatti D, Trainor PA, Sañudo JR, Apaydin N, Şengül G, Albertine KH, Walocha JA, Loukas M, Duparc F, Paulsen F, Del Sol M, Adds P, Hegazy A, Tubbs RS. 2021; Acknowledging the use of human cadaveric tissues in research papers: recommendations from anatomical journal editors. Clin Anat. 34:2–4. DOI: 10.1002/ca.23671. PMID: 32808702.

31. Iwanaga J, Singh V, Takeda S, Ogeng'o J, Kim HJ, Moryś J, Ravi KS, Ribatti D, Trainor PA, Sañudo JR, Apaydin N, Sharma A, Smith HF, Walocha JA, Hegazy AMS, Duparc F, Paulsen F, Del Sol M, Adds P, Louryan S, Fazan VPS, Boddeti RK, Tubbs RS. 2022; Standardized statement for the ethical use of human cadaveric tissues in anatomy research papers: recommendations from Anatomical Journal Editors-in-Chief. Clin Anat. 35:526–8. DOI: 10.1002/ca.23849. PMID: 35218594.
Fig. 1
The accessory submandibular gland (arrow) was noted on left side in 85 years-old at death male cadaver. The gland was located superior to the mylohyoid muscle (the main submandibular gland is removed).
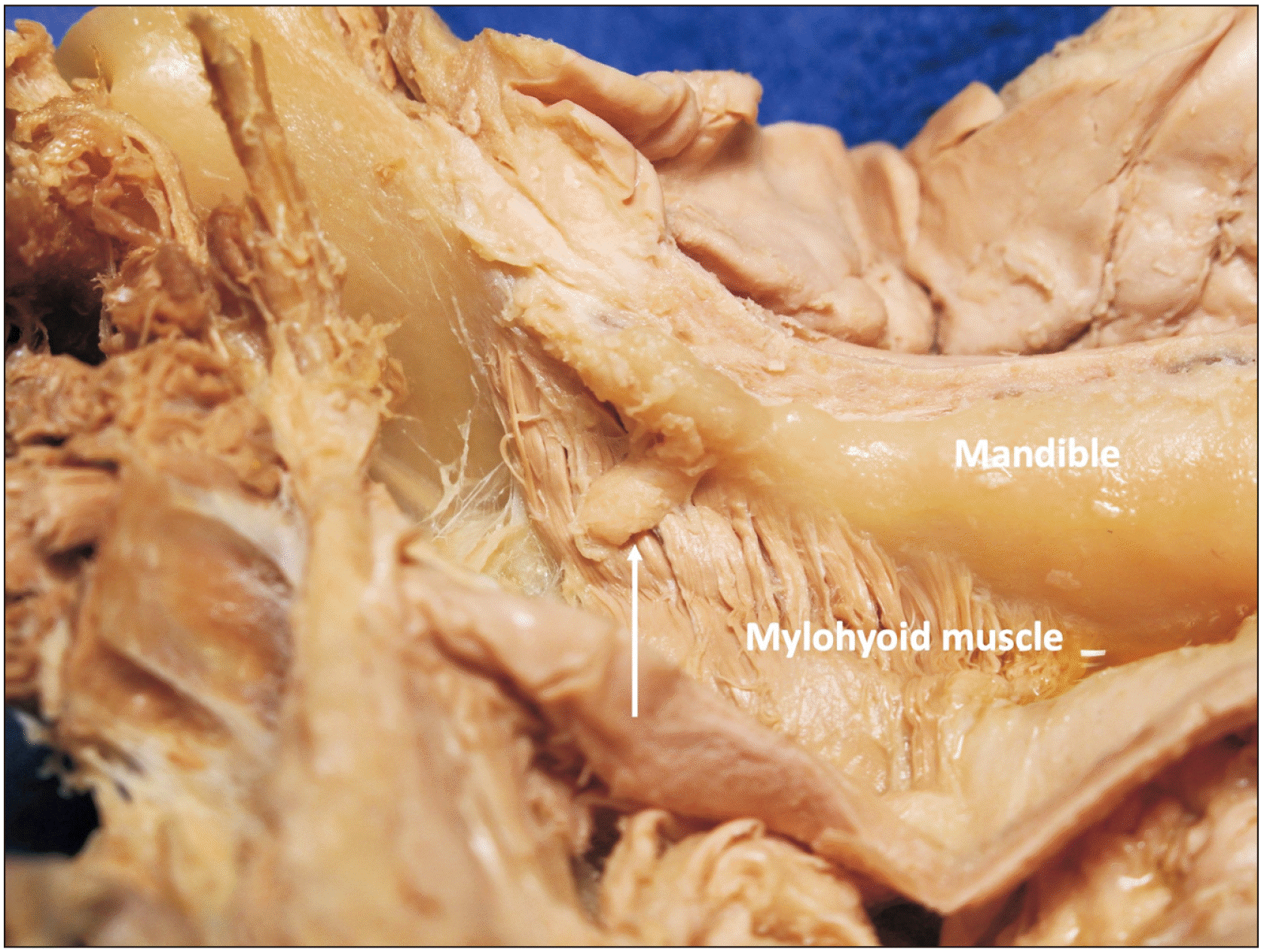
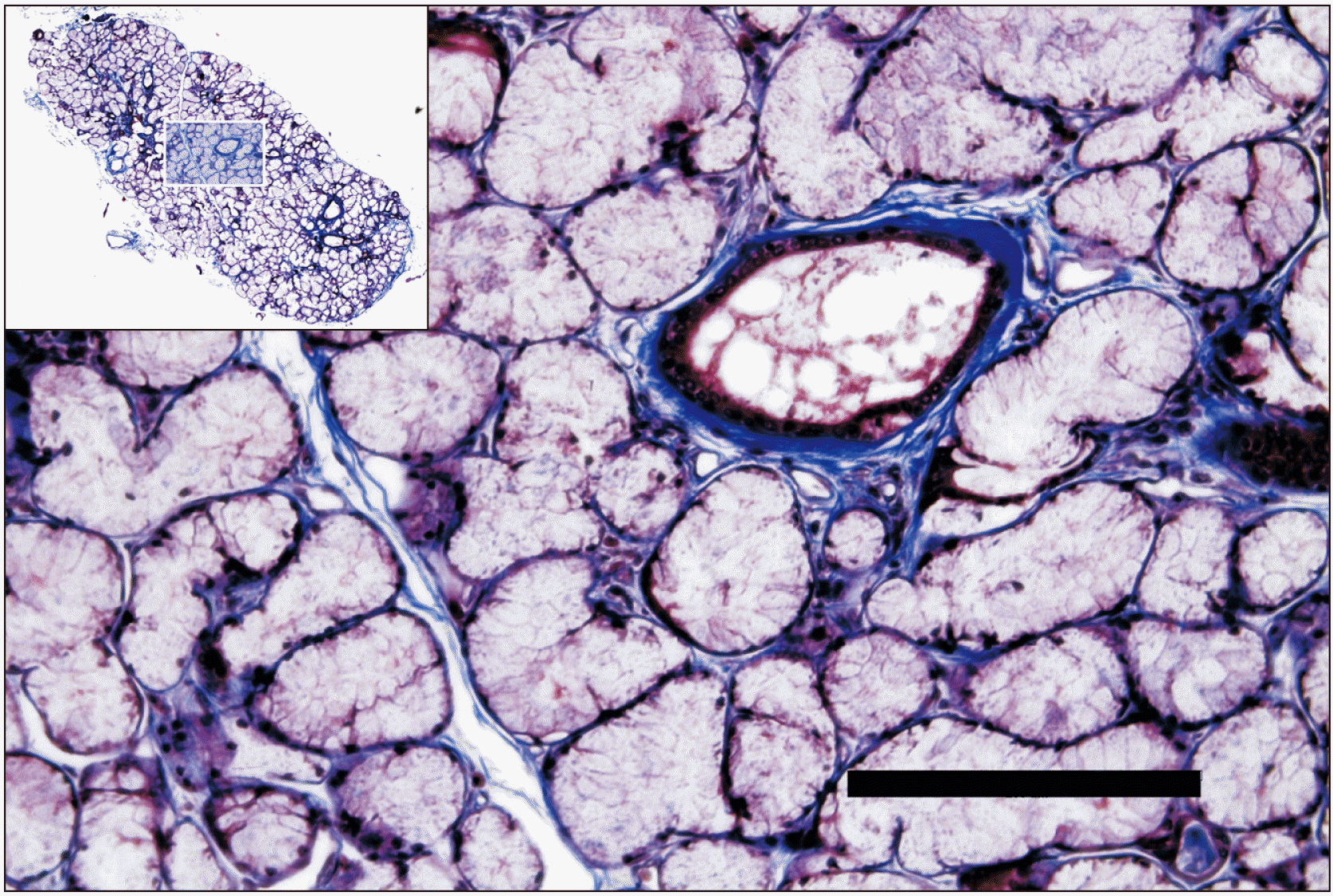
Fig. 2
Masson-trichrome staining of the accessory submandibular gland shown in Fig. 1. The rectangular area in the upper left corner is magnified. Note that the gland tissue is filled with mucinous gland. Scale bar: 200 μm.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download