Introduction
Case Report
Case history
Postmortem CT angiography (PMCTA)
General and pathological findings
 | Fig. 13D VR reconstruction of the heart and great vessels. A dynamic phase of MPMCTA. Right-sided heart, the apex pointing towards the right (star) and AA emerging from the right ventricle. AA, ascending aorta; VR, virtual reconstruction; MPMCTA, multiphase postmortem computed tomographic angiography; 3D, three-dimensional. |
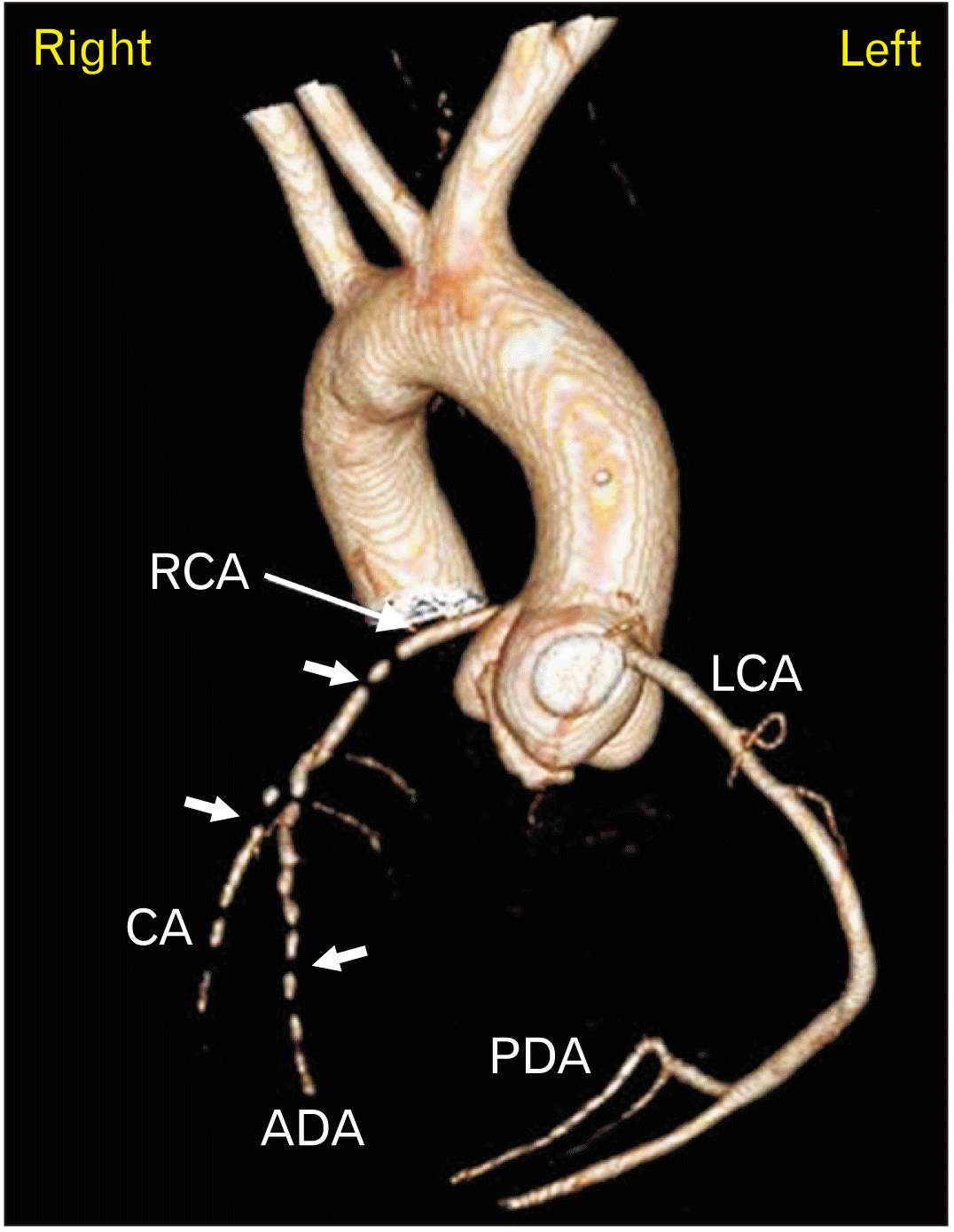 | Fig. 23D VR reconstruction of coronary arteries. Arterial phase of MPMCTA. Note the contrast flow interruptions shown as filling defects (short arrows). VR, virtual reconstruction; MPMCTA, multiphase postmortem computed tomographic angiography; RCA, right coronary artery; LCA, left coronary artery; CA, circumflex artery; ADA, anterior descending artery; PDA, posterior descending artery; 3D, three-dimensional. |
Vascular variations
Right internal mammary artery (IMA)
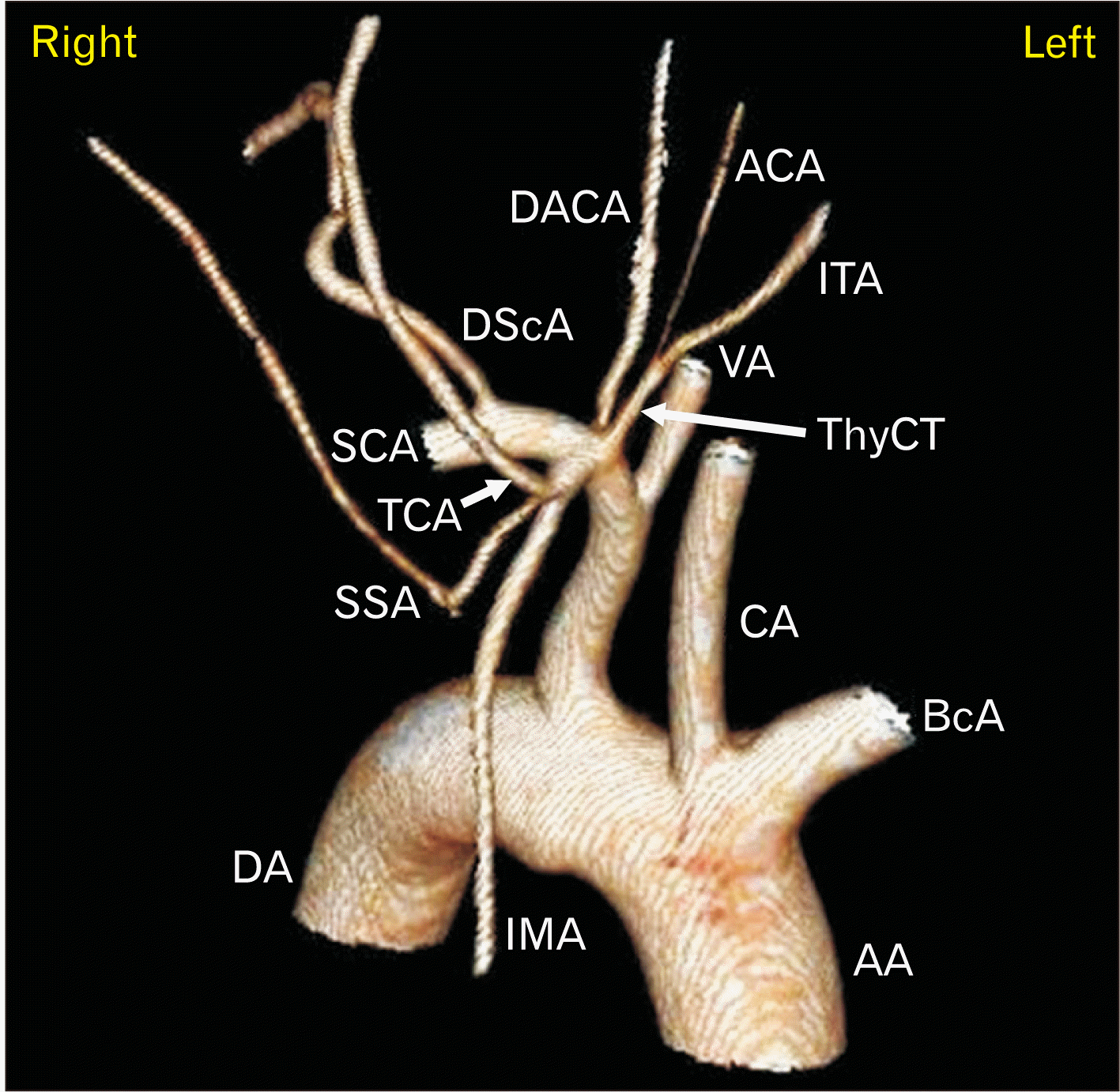 | Fig. 33D VR reconstruction of the right anterior neck. Arterial phase of MPMCTA. Note the common origin of the right IMA with other arterial branches, namely the TCA and SSA. VR, virtual reconstruction; MPMCTA, multiphase postmortem computed tomographic angiography; IMA, internal mammary artery; TCA, transverse cervical artery; SSA, suprascapular artery; AA, ascending aorta; BcA, brachiocephalic artery; CA, common carotid artery; VA, vertebral artery; ThyCT, thyrocervical trunk; ITA, inferior thyroid artery; ACA, ascending cervical artery; DACA, deep ascending cervical artery; DScA, dorsal scapular artery; SCA, subclavian artery; DA, descending aorta; 3D, three-dimensional. |
Thyrocervical trunks
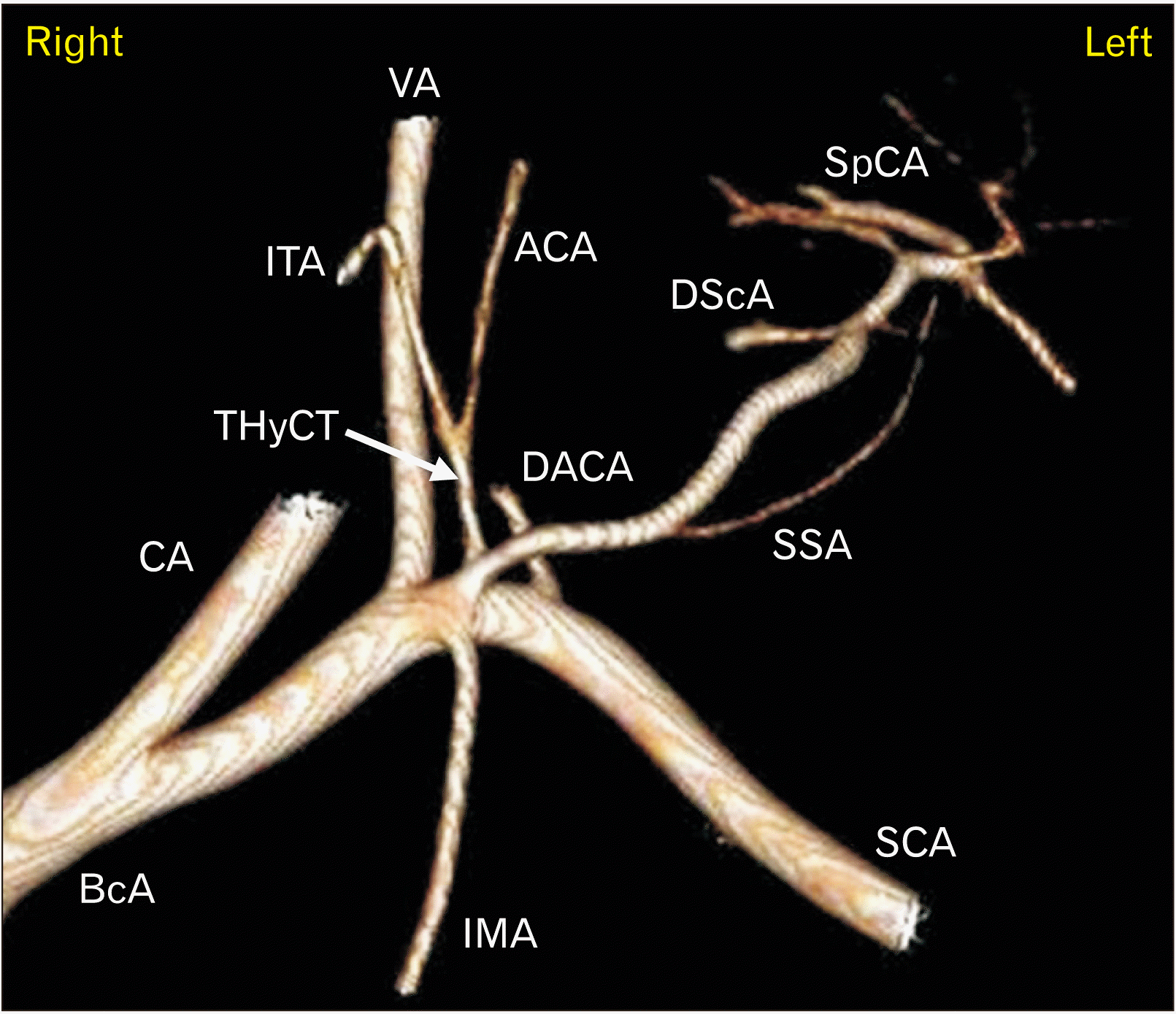 | Fig. 43D VR reconstruction of the left anterior neck. Arterial phase of MPMCTA. Note the common origin of the left ThyCT with another atypical arterial trunk, which is comparatively large. VR, virtual reconstruction; MPMCTA, multiphase postmortem computed tomographic angiography; ThyCT, thyrocervical trunk; BcA, Brachiocephalic artery; CA, carotid artery; VA, vertebral artery; IMA, internal mammary artery; ITA, inferior thyroid artery; ACA, ascending cervical artery; DACA, deep ascending cervical artery; SSA, suprascapular artery; DScA, dorsal scapular artery; SpCA, superficial cervical artery; SCA, subclavian artery; 3D, three-dimensional. |
Drainage of the testicular veins
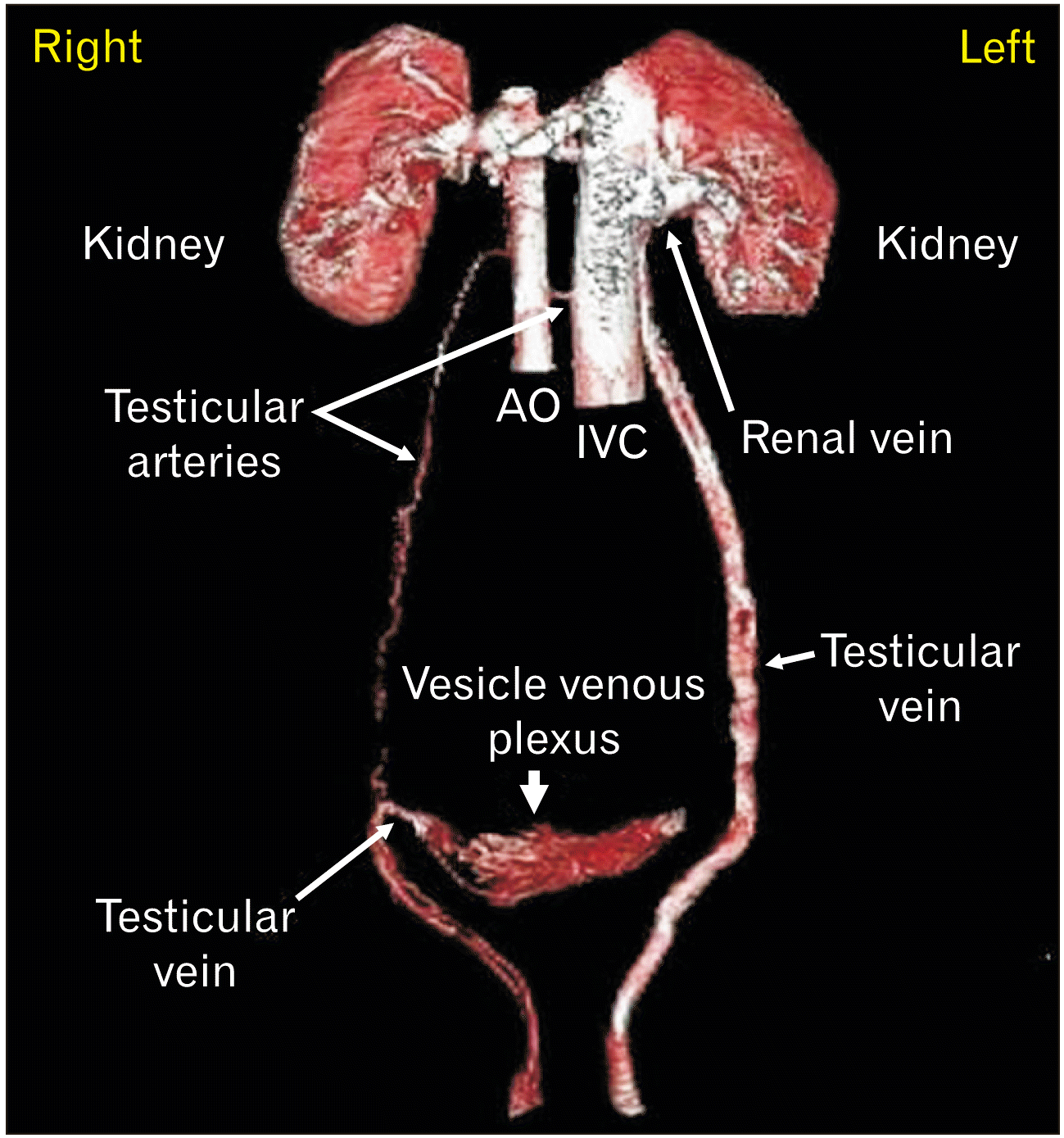 | Fig. 53D VR reconstruction to separate the testicular vessels. A dynamic phase of MPMCTA. Notice dilated & tortuous (varicocele) left testicular vein draining into the left renal vein. Right testicular vein coursing inwards and draining into vesicle venous plexus. Normal testicular arteries. VR, virtual reconstruction; MPMCTA, multiphase postmortem computed tomographic angiography; AO, abdominal aorta; IVC, inferior vena cava; 3D, three-dimensional. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download